In vitro and ex vivo activity of peptide deformylase inhibitors against Mycobacterium tuberculosis H37Rv.
Anshika Sharma, Sadhna Sharma, G K Khuller, A J Kanwar
Index: Int. J. Antimicrob. Agents 34(3) , 226-30, (2009)
Full Text: HTML
Abstract
Bacterial peptide deformylase (PDF) catalyses removal of the N-terminal formyl group of proteins and is essential for protein maturation, growth and survival of bacteria. Thus, PDF appears to be a good antimycobacterial drug target. In the present study, various well-known PDF inhibitors, such as BB-3497, actinonin, 1,10-phenanthroline, hydroxylamine hydrochloride and galardin, were selected to evaluate their inhibitory activity against Mycobacterium tuberculosis. All compounds were found to be active against M. tuberculosis, with MIC(90) values (lowest drug concentration at which 90% of growth was inhibited on the basis of CFU enumeration) ranging from 0.2 mg/L to 74 mg/L. BB-3497 and 1,10-phenanthroline exhibited potent in vitro antimycobacterial activity, and also showed synergism with isoniazid and rifampicin. All compounds showed a bacteriostatic mode of inhibition. Under ex vivo conditions and short-course chemotherapy, BB-3497 and actinonin were found to be significantly active, with BB-3497 exhibiting comparable efficacy to that of isoniazid. Collectively, promising activities of PDF inhibitors such as BB-3497 and actinonin suggest their potential use against M. tuberculosis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
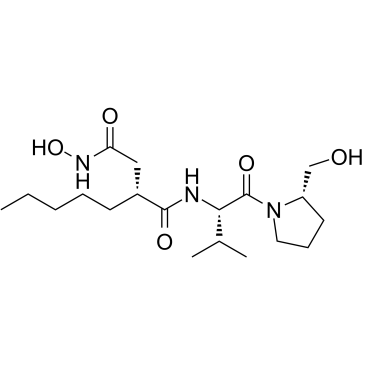 |
ACTINONIN
CAS:13434-13-4 |
C19H35N3O5 |
|
Proteome-wide analysis of the amino terminal status of Esche...
2015-07-01 [Proteomics 15 , 2503-18, (2015)] |
|
Quality control of mitochondrial protein synthesis is requir...
2015-10-26 [J. Cell Biol. 211 , 373-89, (2015)] |
|
Concanavalin-A-induced autophagy biomarkers requires membran...
2012-09-01 [Glycobiology 22(9) , 1245-55, (2012)] |
|
A role for metalloendopeptidases in the breakdown of the gut...
2011-12-01 [Endocrinology 152 , 4630-40, (2011)] |
|
The novel endomorphin degradation blockers Tyr-Pro-DClPhe-Ph...
2010-07-01 [Chem. Biol. Drug Des. 76(1) , 77-81, (2010)] |