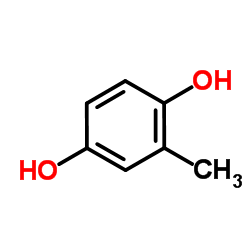
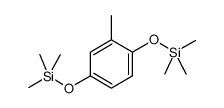
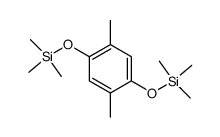
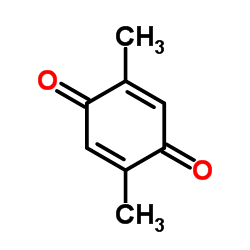
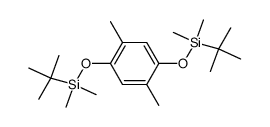
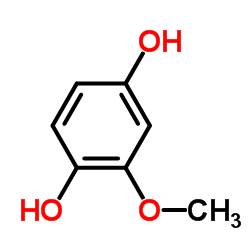
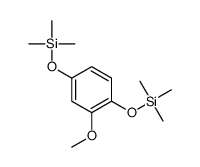
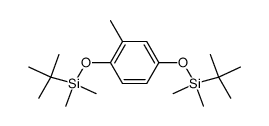
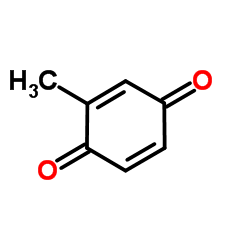
|
~91% |
|
~84% |
|
~94% |
|
~79% |
|
~95% |
|
~88% |
|
~63% |
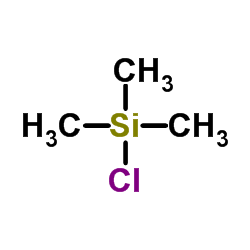
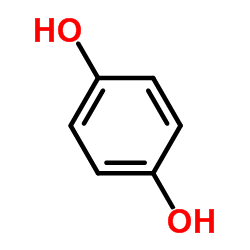

![Silane, [1, 4-phenylenebis (oxy)]bis[trimethyl Structure](https://image.chemsrc.com/caspic/185/2117-24-0.png)