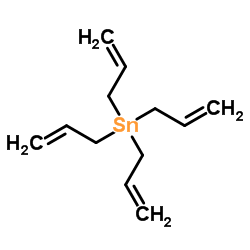
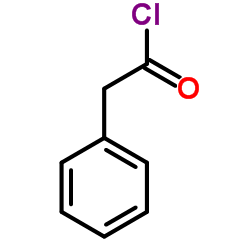
|
~% |
|
~69% |
|
~88% |
|
~19% |
|
~52% |
|
~92% |
|
~96% |
|
~% |
|
~77% |
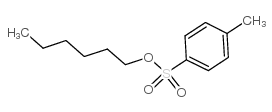
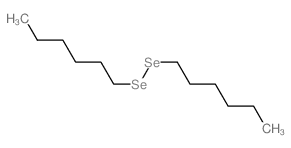
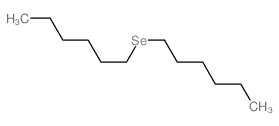
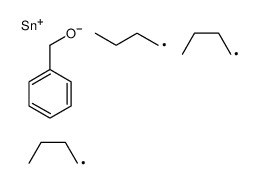


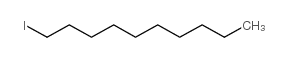


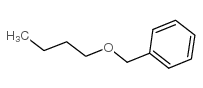

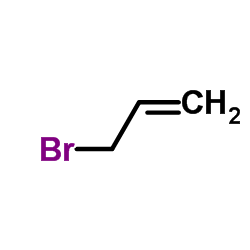
![[(Allyloxy)methyl]benzene Structure](https://image.chemsrc.com/caspic/025/14593-43-2.png)