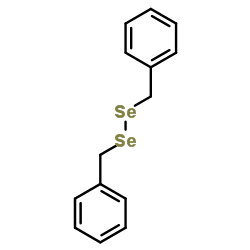
|
~99% |
|
~0% |
|
~0% |
|
~0% |
|
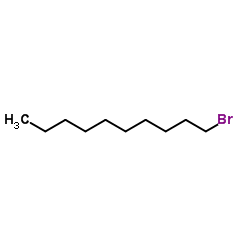
~94% |
|
~0% |
|
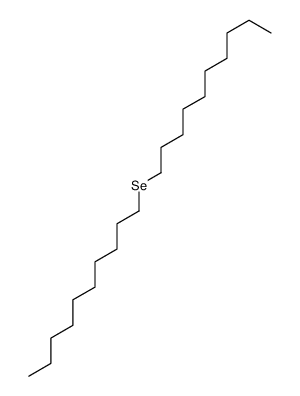
~97% |
|
~0% |
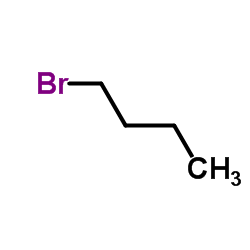



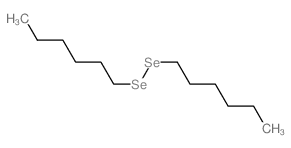
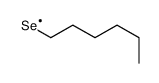
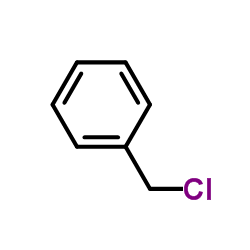
![Benzene,1,1'-[selenobis(methylene)]bis Structure](https://image.chemsrc.com/caspic/234/1842-38-2.png)