Inhibitory effects of plant-derived flavonoids and phenolic acids on malonaldehyde formation from ethyl arachidonate.
Kwang-Geun Lee, Takayuki Shibamoto, Gary R Takeoka, Sung-Eun Lee, Jeong-Han Kim, Byeoung-Soo Park
Index: J. Agric. Food Chem. 51(24) , 7203-7, (2003)
Full Text: HTML
Abstract
The antioxidant activities of naturally occurring plant compounds were measured in a lipid peroxidation system consisting of ethyl arachidonate and Fenton's reagent. Inhibitory effects of 24 plant-derived flavonoids and 5 phenolic acids on malonaldehyde (MA) formation from ethyl arachidonate were examined using gas chromatography (GC) with a nitrogen-phosphorus detector (NPD). Luteolin, which showed the strongest antioxidant activity, inhibited MA formation by 94% and 97% at the levels of 0.5 and 1.0 mM, respectively. The antioxidant activities of the flavones and flavonols decreased in the following order: luteolin > rhamnetin > fisetin > kaempferol > morin > quercetin. Among the flavanones tested, hesperitin, taxifolin, and naringenin exhibited appreciable antioxidant activities (61-84%) at the 1.0 mM level. The inhibitory effect of epigallocatechin gallate (82.5% at the 1.0 mM level) was the strongest among the flavan-3-ols tested. Ferulic acid had the most potent antioxidant activity (74.6% at the 1.0 mM level) of the phenolic acids tested.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
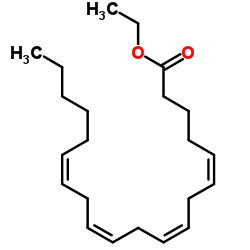 |
Ethyl (5Z,8Z,11Z,14Z)-5,8,11,14-icosatetraenoate
CAS:1808-26-0 |
C22H36O2 |
|
Development of a new immunoassay for the detection of ethyl ...
2014-08-01 [Clin. Chem. Lab Med. 52(8) , 1179-85, (2014)] |
|
Kinetic study of the prooxidant effect of alpha-tocopherol. ...
2009-10-01 [Lipids 44(10) , 935-43, (2009)] |
|
Fatty acid ethyl esters in meconium are associated with poor...
2008-06-01 [J. Pediatr. 152(6) , 788-92, (2008)] |
|
Ethyl arachidonate is the predominant fatty acid ethyl ester...
2003-03-01 [Lipids 38(3) , 269-73, (2003)] |
|
Simultaneous determination of acrolein, malonaldehyde and 4-...
1996-10-01 [Food Chem. Toxicol. 34(10) , 1009-11, (1996)] |