Potent mutagenic potential of 4-methylquinoline: metabolic and mechanistic considerations.
K Saeki, K Takahashi, Y Kawazoe
Index: Biol. Pharm. Bull. 19(4) , 541-6, (1996)
Full Text: HTML
Abstract
4-Methylquinoline (4-MeQ) showed an extraordinarily potent mutagenicity when compared to quinoline and isomeric methylquinolines. The major metabolite of 4-MeQ was 4-hydroxymethylquinoline, which was not mutagenic under the assay condition employed. Deuteration of the methyl group of 4-MeQ resulted in a decrease in the amount of the hydroxymethyl metabolite and an increase in mutagenicity, indicating that hydroxylation of the substituent methyl group is a detoxication process. A 3-chloro derivative of 4-MeQ was proven to be non-mutagenic. 4-Ethyl-quinoline, as well as 4-hydroxymethylquinoline, was much less mutagenic than 4-MeQ. Taking account of the structure-mutagenicity relationship, a possible mechanism is proposed for the potent mutagenic potential of 4-MeQ.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
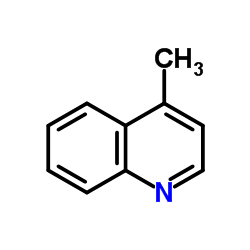 |
lepidine
CAS:491-35-0 |
C10H9N |
|
Time-of-flight accurate mass spectrometry identification of ...
2015-08-01 [Anal. Bioanal. Chem 407 , 6159-70, (2015)] |
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-,...
2014-02-07 [Org. Lett. 16(3) , 864-7, (2014)] |
|
A Programmed DNA Marker Based on Bis(4-ethynyl-1,8-naphthali...
2015-11-09 [Chemistry 21 , 16623-30, (2015)] |
|
Microbial metabolism of quinoline and related compounds. XIX...
1993-07-01 [Biol. Chem. Hoppe-Seyler 374 , 479-488, (1993)] |
|
Carcinogenicity of quinoline, 4- and 8-methylquinoline and b...
1988-07-01 [Food Chem. Toxicol. 26(7) , 625-9, (1988)] |