| Structure | Name/CAS No. | Articles |
|---|---|---|
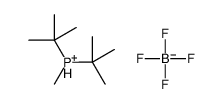 |
di-tert-butylmethylphosphine tetrafluor&
CAS:870777-30-3 |
|
 |
Benzene,1,2,4,5-tetrafluoro-3-methyl
CAS:5230-78-4 |