Direct Aerobic , _-Dehydrogenation of Aldehydes and Ketones with a Pd(TFA)(2)/4,5-Diazafluorenone Catalyst().
Tianning Diao, TylerJ Wadzinski, ShannonS Stahl
Index: Chem. Sci. 3 , 887-891, (2012)
Full Text: HTML
Abstract
The direct , _-dehydrogenation of aldehydes and ketones represents an efficient alternative to stepwise methods to prepare enal and enone products. Here, we describe a new Pd(TFA)(2)/4,5-diazafluorenone dehydrogenation catalyst that overcomes key limitations of previous catalyst systems. The scope includes successful reactivity with pharmaceutically important cyclopentanone and flavanone substrates, as well as acyclic ketones. Preliminary mechanistic studies compare the reactivity of this catalyst to previously reported dehydrogenation catalysts and reveal that cleavage of the -C-H bond of the ketone is the turnover-limiting step of the catalytic mechanism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
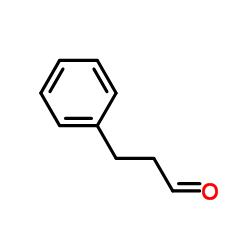 |
3-Phenylpropanal
CAS:104-53-0 |
C9H10O |
|
Well-defined N-heterocyclic carbene silver halides of 1-cycl...
2011-03-07 [Dalton Trans. 40(9) , 2046-52, (2011)] |
|
Rh-catalyzed linear hydroformylation of styrene.
2013-01-07 [Dalton Trans. 42(1) , 137-42, (2013)] |
|
Enantioselective -Chlorination of Aldehydes with Recyclable ...
2011-05-01 [Synlett 3(2010) , 433-436, (2010)] |
|
Total synthesis of (+)-strictifolione.
2003-05-29 [Org. Lett. 5(11) , 1995-7, (2003)] |
|
The arduous way to the egg: follow the nose.
2003-10-13 [Angew. Chem. Int. Ed. Engl. 42(39) , 4729-31, (2003)] |