Organic Letters
2013-04-05
Chiral Brønsted acid catalyzed enantioselective synthesis of anti-homopropargyl alcohols via kinetic resolution-aldehyde allenylboration using racemic allenylboronates.
Andy S Tsai, Ming Chen, William R Roush
Index: Org. Lett. 15(7) , 1568-71, (2013)
Full Text: HTML
Abstract
A chiral phosphoric acid catalyzed kinetic resolution/allenylboration of racemic allenylboronates with aldehydes is described. Allenylboration of aldehydes with 2.8 equiv of allenylboronate (±)-1 in the presence of 10 mol % of catalyst (R)-2 provided anti-homopropargyl alcohols 3 in 83-95% yield with 9:1 to 20:1 diastereoselectivity and 73-95% ee. The catalyst enables the kinetic resolution of the racemic allenylboronate (±)-1 to set the methyl stereocenter and biases the facial attack of the aldehyde to set the stereochemistry of the hydroxyl group in 3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
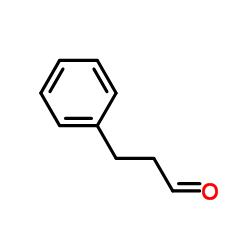 |
3-Phenylpropanal
CAS:104-53-0 |
C9H10O |
Related Articles:
More...
|
Well-defined N-heterocyclic carbene silver halides of 1-cycl...
2011-03-07 [Dalton Trans. 40(9) , 2046-52, (2011)] |
|
Rh-catalyzed linear hydroformylation of styrene.
2013-01-07 [Dalton Trans. 42(1) , 137-42, (2013)] |
|
Enantioselective -Chlorination of Aldehydes with Recyclable ...
2011-05-01 [Synlett 3(2010) , 433-436, (2010)] |
|
Total synthesis of (+)-strictifolione.
2003-05-29 [Org. Lett. 5(11) , 1995-7, (2003)] |
|
The arduous way to the egg: follow the nose.
2003-10-13 [Angew. Chem. Int. Ed. Engl. 42(39) , 4729-31, (2003)] |