Kinetic behavior and interaction of bacampicillin in alginic acid solution at neural pH region.
S Kawashima, Y Yamada, K Honda, T Tabata, M Naito, H Fujiwara
Index: Chem. Pharm. Bull. 37(9) , 2485-90, (1989)
Full Text: HTML
Abstract
Stabilization of bacampicillin (BAPC) in suspension was examined by the addition of alginic acid (Alg). BAPC formed a slightly water-soluble adduct (BAPC-Alg) with Alg, in which BAPC and Alg were presumed to be linked by ionic bonding. However, the suspension of this chemically stable adduct showed a lability to a suspension of BAPC alone; chemically very unstable particles of BAPC base were deposited in the suspension. In contrast, when BAPC-Alg adduct was suspended in 1.0% Alg solution at the same pH region, the precipitation of the particles of BAPC base were not observed. This stabilization is supposed to be due not only to the chemical stability of the adduct, but also to an inhibition of the deposition of an unstable BAPC base particles by Alg.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
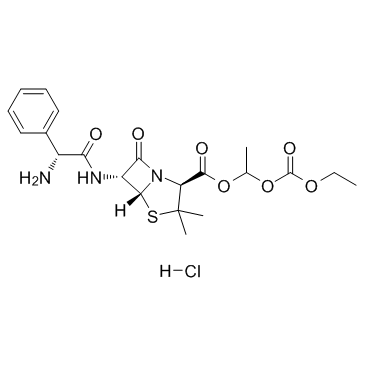 |
Bacampicillin hydrochloride
CAS:37661-08-8 |
C21H28ClN3O7S |
|
Ampicillin and its congener prodrugs in the horse.
1994-01-01 [Br. Vet. J. 150(2) , 173-87, (1994)] |
|
Eudragit E microspheres containing bacampicillin: preparatio...
1991-01-01 [J. Microencapsul. 8(3) , 401-6, (1991)] |
|
Ampicillin concentrations in radicular cysts following a sin...
1993-07-01 [Gen. Pharmacol. 24(4) , 895-8, (1993)] |
|
Pharmacokinetics of bacampicillin in equids.
1995-11-01 [Am. J. Vet. Res. 56(11) , 1486-92, (1995)] |
|
Ampicillin concentrations in human radicular granuloma follo...
1992-01-01 [J. Oral Maxillofac. Surg. 50(1) , 11-3, (1992)] |