Reversible changes in the fluorescence energy transfer accompanying formation of reaction intermediates in probe-labeled (Na+,K+)-ATPase.
K Taniguchi, S Mårdh
Index: J. Biol. Chem. 268(21) , 15588-94, (1993)
Full Text: HTML
Abstract
A preparation of pig kidney Na+,K(+)-ATPase showed changes in fluorescence energy transfer between probes bound to the alpha-subunit. Excitation (305 nm) of an N-(p-(2-benzimidazolyl)phenyl)maleimide (BIPM) probe, which was covalently bound to Cys-964, and excitation (470 nm) of a fluorescein 5'-isothiocyanate (FITC) probe at Lys-501 gave different FITC fluorescence intensity changes at 520 nm in BIPM-FITC doubly labeled enzyme accompanying formation of reaction intermediates. Addition of acetyl phosphate to a Na(+)-bound enzyme (NaE1) to accumulate acetate-sensitive phosphoenzyme (E1P) induced a faster and greater FITC fluorescence decrease when excited at 470 nm than at 305 nm. An oligomycin-sensitive transition of E1P to K(+)-sensitive phosphoenzyme (E2P) was also accompanied by a larger FITC fluorescence decrease when excited at 470 nm. The addition of K+ to [32P]E2P to form a K(+)-bound enzyme (KE2) induced a rapid dephosphorylation (140/s) with both a slow rate of FITC fluorescence increase and a larger fluorescence increase when excited at 470 nm. The addition of Na+ to KE2 induced both a slow increase of FITC fluorescence and a larger fluorescence increase when excited at 470 nm. The data suggest that fluorescence energy transfer from the BIPM to the FITC probe accompanies the processes of migration of Na+ and K+ in the pump molecules. The dynamic fluorescence changes after phosphorylation and dephosphorylation seem to reflect changes in the binding state or the process of migration of these ions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
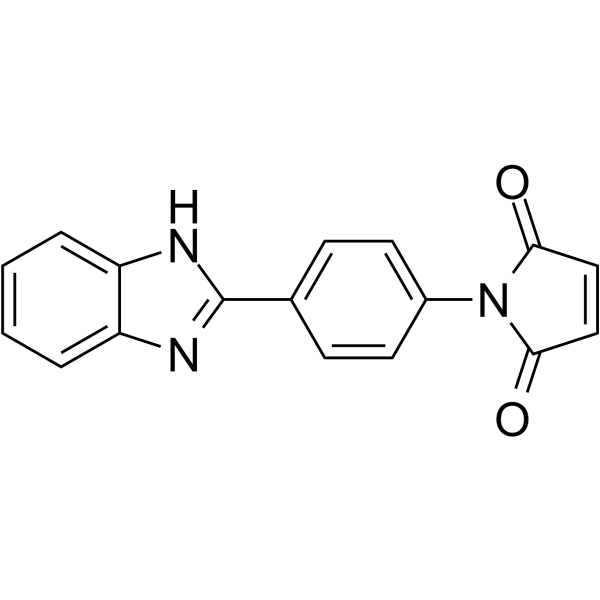 |
N-(4-(2-benzimidazolyl)phenyl)maleimide
CAS:27030-97-3 |
C17H11N3O2 |
|
Conformational change accompanying formation of oligomycin-i...
1991-02-01 [J. Biochem. 109(2) , 299-306, (1991)] |
|
Different susceptibility to phospholipase A2 treatment of th...
1994-03-01 [J. Biochem. 115(3) , 454-62, (1994)] |
|
ATP-induced dynamic fluorescence changes of a N-[p-(2-benzim...
1997-09-01 [J. Biochem. 122(3) , 659-65, (1997)] |
|
[Ouabain binding and the conformational change in Na+, K+ -A...
1985-09-01 [Nippon. Yakurigaku Zasshi. 86(3) , 181-8, (1985)] |
|
Organic fluorescence reagents in the study of enzymes and pr...
1977-03-01 [Angew. Chem. Int. Ed. Engl. 16 , 137, (1977)] |