Fluorescence derivatisation of urinary corticosteroids for high-performance liquid chromatographic analysis.
E Neufeld, R Chayen, N Stern
Index: J. Chromatogr. B. Biomed. Sci. Appl. 718(2) , 273-7, (1998)
Full Text: HTML
Abstract
Corticosteroids containing a C21 primary hydroxyl group were derivatised with 9-anthroyl cyanide. The reagent was prepared as a solution in acetonitrile, containing 0.1% triethylamine, at a concentration of 2 mg/ml. Approximately 1 microg of corticosteroid was reacted with 100 microl of this reagent, at 45 degrees C for 2 h. The fluorescent derivatives were separated by HPLC on a silica column, 250x4.6 mm I.D., by stepwise elution, with a mobile phase of 2-propanol-hexane (2:98) for 20 min, followed by 2-propanol-hexane (7:93) from 20 to 40 min. The fluorescence detector was set to 370-nm excitation and 470-nm emission. The relatively low temperature for derivatisation avoided reaction with secondary hydroxyl groups and also prevented thermal degradation of the corticosteroids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
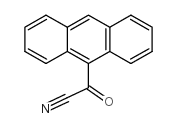 |
9-anthroylnitrile
CAS:85985-44-0 |
C16H9NO |
|
HPLC separation and ultrasensitive optical quantification of...
2015-04-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 986-987 , 123-8, (2015)] |
|
Determination of farnesyl pyrophosphate in dog and human pla...
1997-10-01 [Anal. Biochem. 252(1) , 89-95, (1997)] |
|
Interactions between tissue-type plasminogen activator and e...
1989-10-25 [J. Biol. Chem. 264(30) , 18180-7, (1989)] |
|
Fluorescent high-performance liquid chromatography assay for...
2011-12-01 [Anal. Biochem. 419(1) , 40-5, (2011)] |
|
J. Goto et al.
[Anal. Chim. Acta 147 , 397, (1983)] |