Fungal cleavage of thioether bond found in Yperite.
N Itoh, M Yoshida, T Miyamoto, H Ichinose, H Wariishi, H Tanaka
Index: FEBS Lett. 412(2) , 281-4, (1997)
Full Text: HTML
Abstract
The degradation of thiodiglycol (I) and benzyl sulfide (II) was attempted using Coriolus versicolor and Tyromyces palustris to investigate the potential ability of basidiomycetes to degrade Yperite (bis(2-chloroethyl) sulfide), a mass-produced and stored chemical warfare agent. I was very rapidly degraded by both fungi. The metabolic pathway of II was elucidated, showing that the initial step was the hydrolytic cleavage of the thioether bond to yield benzyl alcohol and benzyl mercaptan. Benzyl alcohol was further oxidized and finally mineralized. Benzyl mercaptan is reversibly converted to benzyl disulfide and also converted to benzyl alcohol. Finally, the effective degradation of bis(2-bromoethyl) sulfide strongly suggests that basidiomycete would be a potential tool for Yperite degradation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
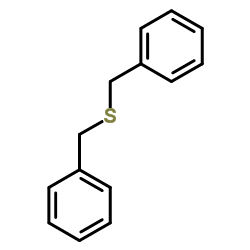 |
benzylsulfide
CAS:538-74-9 |
C14H14S |
|
Singlet oxygen promoted carbon-heteroatom bond cleavage in d...
2007-12-07 [J. Org. Chem. 72(25) , 9582-9, (2007)] |
|
Functional groups and sulfur K-edge XANES spectra: divalent ...
2010-09-09 [J. Phys. Chem. A 114(35) , 9523-8, (2010)] |
|
Dibenzyl sulfide metabolism by white rot fungi.
2003-02-01 [Appl. Environ. Microbiol. 69(2) , 1320-4, (2003)] |
|
[Diffusional system for examination of the solubility and pe...
1980-01-01 [Zentralbl. Bakteriol. Naturwiss. 135(4) , 308-12, (1980)] |
|
A combined theoretical and experimental investigation on the...
2009-12-14 [Chemistry 15(48) , 13417-26, (2009)] |