Butylated hydroxyanisole specifically inhibits tumor necrosis factor-induced cytotoxicity and growth enhancement.
O L Brekke, M R Shalaby, A Sundan, T Espevik, K S Bjerve
Index: Cytokine 4(4) , 269-80, (1992)
Full Text: HTML
Abstract
The effect of commonly used food antioxidants on recombinant tumor necrosis factor alpha (rTNF-alpha)-induced cytotoxicity, growth enhancement and adhesion has been evaluated. Butylated hydroxyanisole (BHA) and 4-hydroxymethyl-2,6-di-t-butylphenol (HBP) were the only two of nine antioxidants that completely inhibited rTNF-alpha-induced cytotoxicity in L929 and WEHI 164 fibrosarcoma cells. Ethoxyquin, propyl gallate and butylated hydroquinone only partially inhibited rTNF-alpha-induced cytotoxicity, while the antioxidants butylated hydroxytoluene (BHT), alpha-tocopherol, ascorbic acid and thiodipropionic acid had minimal effects. The only difference between the molecular structure of the efficient HBP and the non-efficient BHT, is a hydroxymethyl group instead of a hydroxyl group on the phenolic ring. Neither BHA nor BHT inhibited the activation of NF kappa B after 10 or 60 min challenge with rTNF-alpha in L929 cells. BHA also inhibited rTNF-alpha-induced, but not rIL-1 beta-induced growth enhancement in FS-4 fibroblasts. Further, BHA blocked both rTNF-alpha-induced and rIL-1 beta-induced prostaglandin E2 synthesis in FS-4 fibroblasts. BHA inhibited the rTNF-alpha-induced release of arachidonic acid in both FS-4 and L929 cells, suggesting that BHA inhibits cellular phospholipase(s). Neither alpha-tocopherol nor BHA inhibited rTNF-alpha-induced adhesiveness of human endothelial cells. The results indicate that BHA is a specific and potent inhibitor of rTNF-alpha- and rTNF-beta-induced cytotoxicity, as well as of rTNF-alpha-induced growth enhancement.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
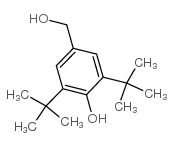 |
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol
CAS:88-26-6 |
C15H24O2 |
|
Ageing of Neurospora crassa. III. Induction of cellular deat...
1976-01-01 [Mech. Ageing Dev. 5(3) , 163-9, (1976)] |
|
The role of low levels of water in the electrochemical oxida...
2011-07-28 [Phys. Chem. Chem. Phys. 13(28) , 12745-54, (2011)] |
|
Down-regulation by butylated hydroxytoluene of the number an...
1995-10-01 [Carcinogenesis 16(10) , 2575-82, (1995)] |
|
The efficiency of antioxidants delivered by liposomal transf...
1997-08-14 [Biochim. Biophys. Acta 1328(1) , 1-12, (1997)] |
|
The antioxidant activity of 3, 5-di-tert-butyl-4-hydroxybenz...
[J. Am. Chem. Soc. 39(3) , 150-55, (1962)] |