|
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![3-[(3AS,4S,7AS)-7A-METHYL-1,5-DIOXOOCTAHYDRO-1H-INDEN-4-YL]PROPIONIC ACID Structure](https://image.chemsrc.com/caspic/145/1944-63-4.png)




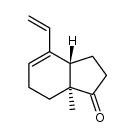
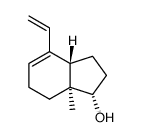

![(3aS,7aS)-4α-ethenyl-1-[(tert-butyldimethylsilyl)oxy]-3a-methyl-3a,6,7,7a-tetrahydro-4-indene Structure](https://image.chemsrc.com/caspic/199/250331-25-0.png)

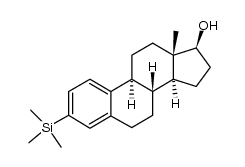
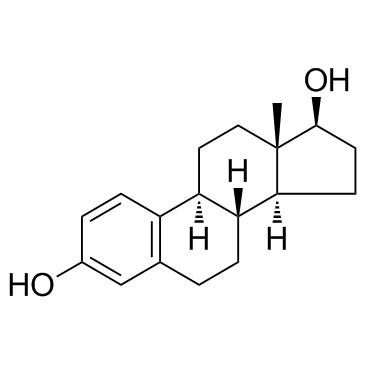
![1-[(tert-butyldimethylsilyl)oxy]-3-trimethylsilyl-9Hβ-estra-1,3,5(10),7-tetraene Structure](https://image.chemsrc.com/caspic/123/250331-27-2.png)
![(1S,2S,5S,9S,13S)-6-[(tert-butyldimethylsilyl)oxy]-5-methyl-15-trimethylsilyl-18-thiatetracyclo[11.4.1.02,10.05,9]-10,14,16-triene-18,18-dioxide Structure](https://image.chemsrc.com/caspic/161/250331-26-1.png)