HPLC determination of benzthiazide in biologic material.
M C Meyer, P Hwang, A B Straughn, K Rotenberg
Index: Biopharm. Drug Dispos. 3(1) , 1-9, (1982)
Full Text: HTML
Abstract
An assay was developed for benzthiazide in plasma, urine and feces, using high performance liquid chromatography (HPLC). A reverse-phase column was employed, with quantitation af 280 nm, using polythiazide as an internal standard. In three of four human subjects who received a 50 mg benzthiazide tablet the plasma concentrations were below the 10 ng ml-1 sensitivity limit of the assay, and the urinary recovery averaged less than one per cent of the dose. One subject received a 50 mg dose as both a tablet and a solution; the urinary recoveries for these two doses were 1.7 and 10.4 per cent, respectively. Fecal samples, obtained from two subjects who received 50 mg tablets, were estimated to contain approximately 80 per cent of the administered dose.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
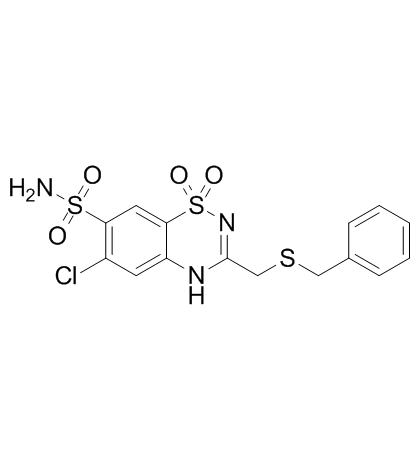 |
Benzthiazide
CAS:91-33-8 |
C15H14ClN3O4S3 |
|
Interactions of diuretics with a neutral temperature-respons...
1999-01-01 [J. Capill. Electrophor. Microchip Technol. 6(5-6) , 163-8, (1999)] |
|
Rational drug repositioning guided by an integrated pharmaco...
2012-01-01 [BMC Syst. Biol. 6 , 80, (2012)] |
|
Rapid screening for diuretic doping agents in urine by C60-a...
1999-09-01 [J. Anal. Toxicol. 23(5) , 337-42, (1999)] |
|
Loop diuretic and anion modification of NEM-induced K transp...
1990-04-01 [Am. J. Physiol. 258(4 Pt 1) , C622-9, (1990)] |
|
Hypercalciuria in hyperprolactinemic rats: effects of benzth...
1986-07-01 [Metab. Clin. Exp. 35(7) , 668-72, (1986)] |