| Structure | Name/CAS No. | Articles |
|---|---|---|
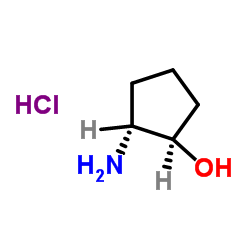 |
trans-2-Amino-cyclopentanol hydrochloride
CAS:31775-67-4 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
trans-2-Amino-cyclopentanol hydrochloride
CAS:31775-67-4 |