Kinetics of oxidation of aliphatic and aromatic thiols by myeloperoxidase compounds I and II.
U Burner, W Jantschko, C Obinger
Index: FEBS Lett. 443 , 290-296, (1999)
Full Text: HTML
Abstract
Myeloperoxidase (MPO) is the most abundant protein in neutrophils and plays a central role in microbial killing and inflammatory tissue damage. Because most of the non-steroidal anti-inflammatory drugs and other drugs contain a thiol group, it is necessary to understand how these substrates are oxidized by MPO. We have performed transient kinetic measurements to study the oxidation of 14 aliphatic and aromatic mono- and dithiols by the MPO intermediates, Compound I (k3) and Compound II (k4), using sequential mixing stopped-flow techniques. The one-electron reduction of Compound I by aromatic thiols (e.g. methimidazole, 2-mercaptopurine and 6-mercaptopurine) varied by less than a factor of seven (between 1.39 +/- 0.12 x 10(5) M(-1) s(-1) and 9.16 +/- 1.63 x 10(5) M(-1) s(-1)), whereas reduction by aliphatic thiols was demonstrated to depend on their overall net charge and hydrophobic character and not on the percentage of thiol deprotonation or redox potential. Cysteamine, cysteine methyl ester, cysteine ethyl ester and alpha-lipoic acid showed k3 values comparable to aromatic thiols, whereas a free carboxy group (e.g. cysteine, N-acetylcysteine, glutathione) diminished k3 dramatically. The one-electron reduction of Compound II was far more constrained by the nature of the substrate. Reduction by methimidazole, 2-mercaptopurine and 6-mercaptopurine showed second-order rate constants (k4) of 1.33 +/- 0.08 x 10(5) M(-1) s(-1), 5.25 +/- 0.07 x 10(5) M(-1) s(-1) and 3.03 +/- 0.07 x 10(3) M(-1) s(-1). Even at high concentrations cysteine, penicillamine and glutathione could not reduce Compound II, whereas cysteamine (4.27 +/- 0.05 x 10(3) M(-1) s(-1)), cysteine methyl ester (8.14 +/- 0.08 x 10(3) M(-1) s(-1)), cysteine ethyl ester (3.76 +/- 0.17 x 10(3) M(-1) s(-1)) and alpha-lipoic acid (4.78 +/- 0.07 x 10(4) M(-1) s(-1)) were demonstrated to reduce Compound II and thus could be expected to be oxidized by MPO without co-substrates.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
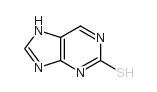 |
2H-Purine-2-thione,3,9-dihydro
CAS:28128-19-0 |
C5H4N4S |
|
Analysis of phenolic compounds in the dissolved and suspende...
2015-08-01 [Environ. Sci. Pollut. Res. Int. 22 , 11966-74, (2015)] |
|
Rate Coefficients for the Gas-Phase Reactions of Hydroxyl Ra...
2015-06-18 [J. Phys. Chem. A 119 , 6179-87, (2015)] |
|
6-Mercaptopurine decreases the Bcl-2/Bax ratio and induces a...
1997-03-01 [Mol. Pharmacol. 51 , 414-421, (1997)] |
|
Nature and position of functional group on thiopurine substr...
2007-02-01 [Biochemistry. (Mosc.) 72 , 170-177, (2007)] |