Interaction and thermal stability of fluorescent labeled derivatives of thrombin and antithrombin III.
D H Atha, S A Brew, K C Ingham
Index: Biochim. Biophys. Acta 785(1-2) , 1-6, (1984)
Full Text: HTML
Abstract
Derivatives of human thrombin and antithrombin III with fluorescent labels covalently attached to their carbohydrate moieties were prepared by reaction of periodate-oxidized proteins with amino derivatives of dansyl, fluorescein and pyrene. The labeled derivatives retained full biological activity, including their ability to form stable enzyme-inhibitor complexes, a reaction whose rate could be monitored by the increase in fluorescence polarization. When the dansyl-labeled derivatives were heated, they exhibited sigmoidal increases in polarization with midpoints near 50 degrees C for thrombin and 60 degrees C for antithrombin III. By contrast, a complex between antithrombin III and dansyl-thrombin showed no change in polarization up to 70 degrees C, suggesting that the individual components are more stable in the complex. These studies show that fluorescent labels attached to carbohydrate moieties of glycoproteins provide convenient probes for monitoring conformational changes and protein-protein interactions with minimum interference by the probe.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
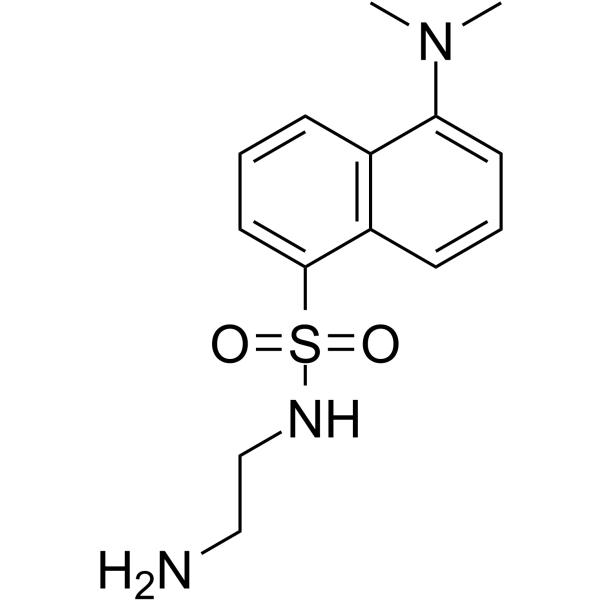 |
Dansyl Ethylenediamine
CAS:35060-08-3 |
C14H19N3O2S |
|
Fluorescent labeling of the carbohydrate moieties of human c...
1981-09-29 [Biochim. Biophys. Acta 670(2) , 181-9, (1981)] |
|
Fluorescent molecularly imprinted polymer thin films for spe...
2013-10-15 [Biosens. Bioelectron. 48 , 113-9, (2013)] |
|
Estimation of affinities of ligands in mixtures via magnetic...
2011-01-01 [BMC Biotechnol. 11 , 44, (2011)] |
|
Conformational changes in subdomain 2 of G-actin: fluorescen...
1995-11-01 [Biophys. J. 69(5) , 2024-32, (1995)] |
|
Myosin-induced changes in F-actin: fluorescence probing of s...
1996-03-01 [Biophys. J. 70(3) , 1439-46, (1996)] |