β-Lapachone analogs with enhanced antiproliferative activity
Carla Ríos-Luci, Evelyn L. Bonifazi, Leticia G. León, Juan C. Montero, Gerardo Burton, Atanasio Pandiella, Rosana I. Misico, José M. Padrón
Index: Eur. J. Med. Chem. 53 , 264-74, (2012)
Full Text: HTML
Abstract
In this study, we describe the synthesis of a series of α- and β-lapachone containing hydroxyl or methoxyl groups on the benzene ring, by means of the selective acid promoted cyclization of the appropriate lapachol analog. The evaluation of the antiproliferative activity in human solid tumor cell lines provided 7-hydroxy-β-lapachone as lead with enhanced activity over the parent drug β-lapachone. Cell cycle studies, protein expression experiments, and reactive oxygen species analysis revealed that, similarly to β-lapachone, ROS formation and DNA damage are critical factors in the cellular toxicity of 7-hydroxy-β-lapachone.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
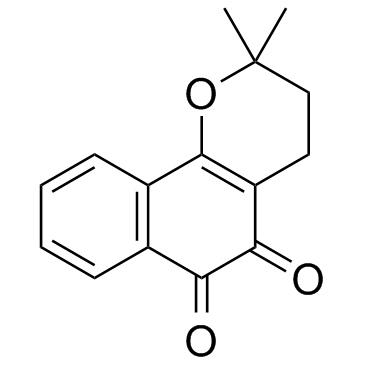 |
Beta-Lapachone
CAS:4707-32-8 |
C15H14O3 |
|
Light effect on the stability of β-lapachone in solution: pa...
2011-09-01 [J. Pharm. Pharmacol. 63(9) , 1156-60, (2011)] |
|
Temperature-sensitive gels for intratumoral delivery of β-la...
2012-01-01 [ScientificWorldJournal 2012 , 126723, (2012)] |
|
Ultrastructural analysis of β-lapachone-induced surface memb...
2014-07-01 [Exp. Parasitol. 142 , 83-90, (2014)] |
|
Mechanistic studies of cancer cell mitochondria- and NQO1-me...
2014-12-15 [Toxicol. Appl. Pharmacol. 281(3) , 285-93, (2014)] |
|
β-lapachone accelerates the recovery of burn-wound skin.
2011-07-01 [Histol. Histopathol. 26(7) , 905-14, (2011)] |