NMR spectroscopic evidence for the intermediacy of XeF(3)(-) in XeF(2)/F(-) exchange, attempted syntheses and thermochemistry of XeF(3)(-) salts, and theoretical studies of the XeF(3)(-) anion.
Neil Vasdev, Matthew D Moran, Heikki M Tuononen, Raman Chirakal, Reijo J Suontamo, Alex D Bain, Gary J Schrobilgen
Index: Inorg. Chem. 49(19) , 8997-9004, (2010)
Full Text: HTML
Abstract
The existence of the trifluoroxenate(II) anion, XeF(3)(-), had been postulated in a prior NMR study of the exchange between fluoride ion and XeF(2) in CH(3)CN solution. The enthalpy of activation for this exchange, ΔH(⧧), has now been determined by use of single selective inversion (19)F NMR spectroscopy to be 74.1 ± 5.0 kJ mol(-1) (0.18 M) and 56.9 ± 6.7 kJ mol(-1) (0.36 M) for equimolar amounts of [N(CH(3))(4)][F] and XeF(2) in CH(3)CN solvent. Although the XeF(3)(-) anion has been observed in the gas phase, attempts to prepare the Cs(+) and N(CH(3))(4)(+) salts of XeF(3)(-) have been unsuccessful, and are attributed to the low fluoride ion affinity of XeF(2) and fluoride ion solvation in CH(3)CN solution. The XeF(3)(-) anion would represent the first example of an AX(3)E(3) valence shell electron pair repulsion (VSEPR) arrangement of electron lone pair and bond pair domains. Fluorine-19 exchange between XeF(2) and the F(-) anion has also been probed computationally using coupled-cluster singles and doubles (CCSD) and density functional theory (DFT; PBE1PBE) methods. The energy-minimized geometry of the ground state shows that the F(-) anion is only weakly coordinated to XeF(2) (F(2)Xe---F(-); a distorted Y-shape possessing C(s) symmetry), while the XeF(3)(-) anion exists as a first-order transition state in the fluoride ion exchange mechanism, and is planar and Y-shaped (C(2v) symmetry). The molecular geometry and bonding of the XeF(3)(-) anion has been described and rationalized in terms of electron localization function (ELF) calculations, as well as the VSEPR model of molecular geometry. Quantum-chemical calculations, using the CCSD method and a continuum solvent model for CH(3)CN, accurately reproduced the transition-state enthalpy observed by (19)F NMR spectroscopy, and showed a negative but negligible enthalpy for the formation of the F(2)Xe---F(-) adduct in this medium.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
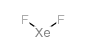 |
Xenon difluoride
CAS:13709-36-9 |
F2Xe |
|
Synthesis of α,α-difluoroethyl aryl and heteroaryl ethers.
2012-08-03 [Org. Lett. 14(15) , 3944-7, (2012)] |
|
Fluorination with XeF(2).(1) 44. Effect of Geometry and Hete...
2001-01-01 [J. Org. Chem. 63 , 878, (1998)] |
|
Xenon difluoride induced aryl iodide reductive elimination: ...
2008-01-07 [Inorg. Chem. 47(1) , 5-7, (2008)] |
|
On the preparation of fluorine-18 labelled XeF(2) and chemic...
2002-10-30 [J. Am. Chem. Soc. 124(43) , 12863-8, (2002)] |
|
Pulsed excimer laser angioplasty of human cadaveric arteries...
1986-02-01 [J. Vasc. Surg. 3(2) , 284-7, (1986)] |