Starch safety in resuscitation - when will we ever learn?
Andy Parrish, Marc Blockman
Index: S. Afr. Med. J. 103(6) , 365-7, (2013)
Full Text: HTML
Abstract
Recent trials have failed to demonstrate a survival benefit from the use of hydroxyethyl starches (HES) as a colloid in fluid resuscitation and have raised concerns of renal harm. In severe sepsis, there is a concerning signal of increased mortality. New high-quality systematic reviews consistently demonstrate a statistically non-significant relative risk of death of 1.08 - 1.10 and a significant 25% increased chance of requiring renal replacement therapy. The HES literature contains many industry-affiliated reviews of indifferent quality. Traditional efficacy confidence limits may warrant re-evaluation when considering these harms. Newer formulations of HES and more focused indications for use show benefit on surrogate endpoints, but these trials are currently underpowered to ensure safety.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
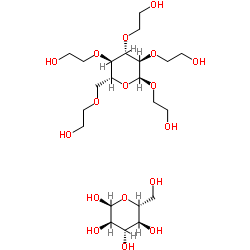 |
Hydroxyethyl starch
CAS:9005-27-0 |
C22H44O17 |
|
Uncontrolled hemorrhagic shock results in a hypercoagulable ...
2013-07-01 [J. Trauma Acute Care Surg. 75(1) , 129-34, (2013)] |
|
Time to assess alternatives to hydroxyethyl starch to use in...
2013-01-01 [BMJ 347 , f4651, (2013)] |
|
Physiological levels of A-, B- and C-type natriuretic peptid...
2013-05-01 [Basic Res. Cardiol. 108(3) , 347, (2013)] |
|
Europe recommends ban on common plasma substitute.
2013-06-01 [BMJ 346 , f3942, (2013)] |
|
Meta-analyses of hydroxyethyl starch for volume resuscitatio...
2013-06-05 [JAMA 309(21) , 2209, (2013)] |