Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia.
Alan Lankford, Roberta Rogowski, Beal Essink, Elizabeth Ludington, H Heith Durrence, Thomas Roth
Index: Sleep Med. 13(2) , 133-8, (2012)
Full Text: HTML
Abstract
The efficacy and safety of doxepin (DXP), a histamine H(1) receptor antagonist, was evaluated in elderly adults with sleep maintenance insomnia.This was a randomized, double-blind, placebo-controlled outpatient trial. Elderly adults meeting DSM-IV-TR criteria for primary insomnia were randomized to four weeks of nightly treatment with either DXP 6 mg (N=130) or placebo (PBO; N=124). Efficacy was assessed using patient self-report instruments and clinician ratings. Patient-reported endpoints included subjective total sleep time (sTST), subjective wake after sleep onset (sWASO), latency to sleep onset (LSO), sleep quality, and a Patient Global Impression scale (PGI). The primary endpoint was sTST at week 1.DXP 6 mg produced significantly more sTST and less sWASO at week 1 (both p-values <0.0001) than PBO. These significant improvements versus placebo were maintained at weeks 2-4 (all p-values <0.05). There were no significant differences in LSO for DXP 6 mg versus PBO. DXP 6 mg significantly improved sleep quality (weeks 1, 3, and 4, p<0.05) and several outcome-related parameters, including several items on the PGI, the severity and improvement items of the Clinician Global Impression scale (CGI; weeks 1 and 2) and the Insomnia Severity Index (ISI; weeks 1-4), all versus PBO. There were no reports of anticholinergic effects (e.g., dry mouth) or memory impairment. The safety profile of DXP 6 mg was comparable to that of PBO.In elderly adults with insomnia, DXP 6 mg produced significant improvements in sleep maintenance, sleep duration, and sleep quality endpoints that were sustained throughout the trial. These data suggest that DXP 6 mg is effective for treating sleep maintenance insomnia and is well-tolerated in elderly adults with chronic primary insomnia.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
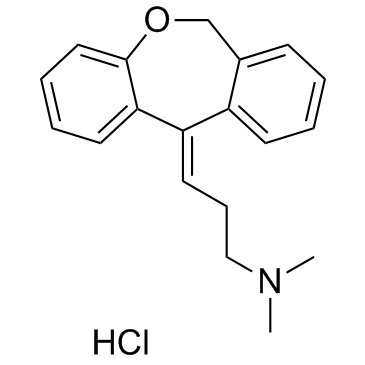 |
Doxepin hydrochloride
CAS:1229-29-4 |
C19H22ClNO |
|
Differential thermodynamic driving force of first- and secon...
2014-09-15 [Biochem. Pharmacol. 91(2) , 231-41, (2014)] |
|
Transbuccal delivery of doxepin: studies on permeation and h...
2014-12-30 [Int. J. Pharm. 477(1-2) , 650-4, (2014)] |
|
[Prolonged toxic coma and anisocoria secondary to doxepin, l...
2012-01-01 [Prz. Lek. 69(8) , 624-6, (2012)] |
|
Analgesic effects of antidepressants alone and after their l...
2014-06-01 [Pharmacol. Rep. 66(3) , 459-65, (2014)] |
|
Transdermal delivery of imipramine and doxepin from newly oi...
2013-03-01 [Colloids Surf. B Biointerfaces 103 , 558-65, (2013)] |