Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia.
Andrew D Krystal, Alan Lankford, H Heith Durrence, Elizabeth Ludington, Philip Jochelson, Roberta Rogowski, Thomas Roth
Index: Sleep 34(10) , 1433-42, (2011)
Full Text: HTML
Abstract
To evaluate the efficacy and safety of doxepin (DXP) 3 mg and 6 mg in adults diagnosed with primary insomnia.The study was a randomized, double-blind, parallel-group, placebo-controlled trial. Patients meeting DSM-IV-TR criteria for primary insomnia were randomized to 35 days of nightly treatment with DXP 3 mg (n=75), DXP 6 mg (n=73), or placebo (PBO; n=73), followed by 2 nights of single-blind PBO to evaluate discontinuation (DC) effects. Efficacy was assessed using polysomnography (PSG) and patient reports. Efficacy data were examined for Night (N) 1, N15, and N29. Safety assessments were conducted throughout the study.Compared with PBO, DXP 3 and 6 mg significantly improved wake time after sleep onset (WASO) on N1 (3 mg and 6 mg; P<0.0001), N15 (3 mg P=0.0025; 6 mg P=0.0009), and N29 (3 mg P=0.0248; 6 mg P=0.0009), latency to persistent sleep (LPS) on N1 (3 mg P=0.0047; 6 mg P=0.0007), and total sleep time (TST) on N1 (3 mg and 6 mg P<0.0001), N15 (6 mg P=0.0035), and N29 (3 mg P=0.0261; 6 mg P<0.0001). In terms of early morning awakenings, DXP 3 and 6 mg demonstrated significant improvements in SE in the final quarter of the night on N1, N15, and N29, with the exception of 3 mg on N29 (P=0.0691). Rates of discontinuation were low, and the safety profiles were comparable across the 3 treatment groups. There were no significant next-day residual effects, and there were no spontaneous reports of memory impairment, complex sleep behaviors, anticholinergic effects, weight gain, or increased appetite. Additionally, there was no evidence of rebound insomnia after DXP discontinuation.Five weeks of nightly administration of DXP 3 mg and 6 mg to adults with chronic primary insomnia resulted in significant and sustained improvements in sleep maintenance and early morning awakenings (with the exception of SE in the final quarter of the night on N29 for 3 mg [P=0.0691]). These sleep improvements were not accompanied by next-day residual effects or followed by rebound insomnia or withdrawal effects upon discontinuation. These findings confirm the unique profile of sleep maintenance efficacy and safety of DXP observed in prior studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
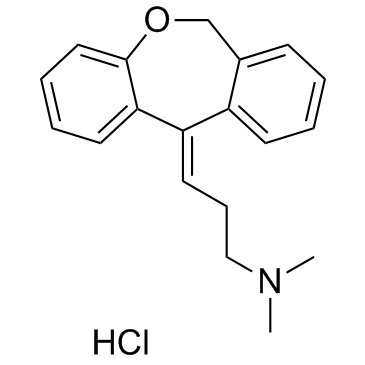 |
Doxepin hydrochloride
CAS:1229-29-4 |
C19H22ClNO |
|
Differential thermodynamic driving force of first- and secon...
2014-09-15 [Biochem. Pharmacol. 91(2) , 231-41, (2014)] |
|
Transbuccal delivery of doxepin: studies on permeation and h...
2014-12-30 [Int. J. Pharm. 477(1-2) , 650-4, (2014)] |
|
[Prolonged toxic coma and anisocoria secondary to doxepin, l...
2012-01-01 [Prz. Lek. 69(8) , 624-6, (2012)] |
|
Analgesic effects of antidepressants alone and after their l...
2014-06-01 [Pharmacol. Rep. 66(3) , 459-65, (2014)] |
|
Transdermal delivery of imipramine and doxepin from newly oi...
2013-03-01 [Colloids Surf. B Biointerfaces 103 , 558-65, (2013)] |