Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the delta log P parameter of seiler.
M H Abraham, H S Chadha, G S Whiting, R C Mitchell
Index: J. Pharm. Sci. 83 , 1085, (1994)
Full Text: HTML
Abstract
A general linear solvation energy equation has been used to analyze published partition coefficients in the systems water-octanol (613 solutes), water-hexadecane (370 solutes), water-alkane (200 solutes), and water-cyclohexane (170 solutes). The descriptors used in the equation are R2, an excess molar refraction; phi H2, the solute dipolarity/polarizability; sigma alpha H2 and sigma beta H2, the effective solute hydrogen-bond acidity and basicity; and Vx, the characteristic volume of McGowan. It is shown that the water-octanol partition coefficient is dominated by solute hydrogen-bond basicity, which favors water, and by solute size, which favors octanol, but solute excess molar refraction and dipolarity/polarizability are also significant. For the water-alkane partition coefficients, the same factors are at work, together with solute hydrogen-bond acidity as a major influence that favors water. An analysis of 288 delta log P values shows that solute hydrogen-bond acidity is the major factor but that solute hydrogen-bond basicity and, to a lesser extent, solute dipolarity/polarizability and size are also significant factors that influence the delta log P parameter.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
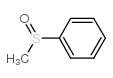 |
methyl phenyl sulfoxide
CAS:1193-82-4 |
C7H8OS |
|
Murraya koenigii leaf extract inhibits proteasome activity a...
2013-01-01 [BMC Complement Altern. Med. 13 , 7, (2013)] |
|
Resolution of racemic sulfoxides with high productivity and ...
2011-01-01 [Bioresour. Technol. 102(2) , 1537-42, (2011)] |
|
Biocatalytic oxidation by chloroperoxidase from Caldariomyce...
2009-11-21 [Org. Biomol. Chem. 7 , 4604-4610, (2009)] |
|
Synthesis, characterization, reactivity, and catalytic poten...
2006-07-24 [Inorg. Chem. 45(15) , 5924-37, (2006)] |
|
Asymmetric synthesis of the potent HIV-protease inhibitor, n...
2010-01-15 [J. Org. Chem. 75(2) , 498-501, (2010)] |