| Structure | Name/CAS No. | Articles |
|---|---|---|
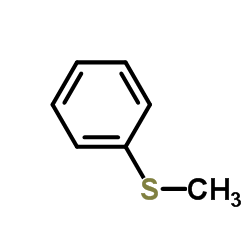 |
Thioanisole
CAS:100-68-5 |
|
 |
Chloroperoxidase, from Caldariomyces fumago
CAS:9055-20-3 |
|
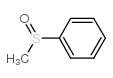 |
methyl phenyl sulfoxide
CAS:1193-82-4 |