[Salts of 5-methylpyrazole-3-carboxylic acid with basically substituted adenine derivatives. Identification and lipolysis inhibitory activity (author's transl)].
K Credner, M Tauscher, L Jozić, G Brenner
Index: Arzneimittelforschung 31(12) , 2096-100, (1981)
Full Text: HTML
Abstract
5-Methylpyrazole-3-carboxylic acid forms salts with basically substituted adenine bases of type (4) or (5), which have significantly more lipolysis inhibitory activity than the free acid itself. The substance tested, 6-amino-9-[2-hydroxy-3-(N-methyl-N-2-hydroxyethyl)-aminopropyl]-purine-5-methyl pyrazole-3-carboxylate (16), causes a significant reduction of serum FFA levels in fasted rats, even at the very low dosage of 0.150 mg/kg b.w., whereas the free 5-methylpyrazole-3-carboxylic-acid shows significant activity in this model only at doses of 2 mg/kg b.w. and above. The salt also shows similar activity on serum triglycerides. The effect on FFA, triglyceride and cholesterol levels, respectively, in fasted rats lasts for at least 8 h.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
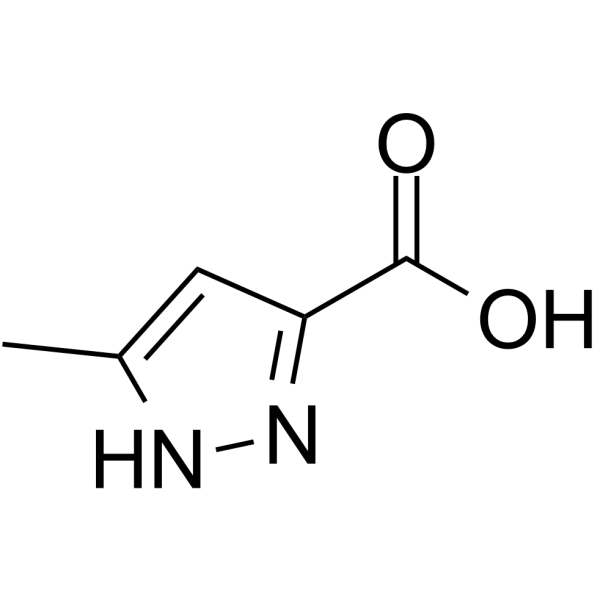 |
5-Methyl-1H-pyrazole-3-carboxylic acid
CAS:402-61-9 |
C5H6N2O2 |
|
Changes in the plasma concentrations of D-kynurenine and kyn...
2010-11-01 [Chirality 22 , 901-916, (2010)] |
|
A series of D-amino acid oxidase inhibitors specifically pre...
2011-01-01 [J. Pharmacol. Exp. Ther. 336 , 282-293, (2011)] |
|
In vitro and in vivo pharmacological profile of AS057278, a ...
2008-03-01 [Eur. Neuropsychopharmacol. 18(3) , 200-14, (2008)] |
|
Plasma prostaglandin levels in rats with diabetes mellitus a...
1982-11-01 [Diabetes 31(11) , 994-1001, (1982)] |
|
Commentary: genome-based CNS drug discovery: D-amino acid ox...
2009-12-01 [Biochem. Pharmacol. 78(11) , 1360-5, (2009)] |