Inhibition of GTP cyclohydrolase I by pterins.
R S Shen, A Alam, Y X Zhang
Index: Biochim. Biophys. Acta 965(1) , 9-15, (1988)
Full Text: HTML
Abstract
Pterins inhibit rat liver GTP cyclohydrolase I activity noncompetitively. Reduced pterins, such as 7,8-dihydro-D-neopterin, (6R,S)-5,6,7,8-tetrahydro-D-neopterin, 7,8-dihydro-L-biopterin, (6R)-5,6,7,8-tetrahydro-L-biopterin, L-sepiapterin, and DL-6-methyl-5,6,7,8-tetrahydropterin are approximately 12-times more potent as inhibitors than are oxidized pterins, such as D-neopterin, L-biopterin, and isoxanthopterin. They are also 12-times more potent than folates, such as folic acid, dihydrofolic acid, (+/-)-L-tetrahydrofolic acid, and aminopterin. The Ki values for 7,8-dihydro-D-neopterin, 7,8-dihydro-L-biopterin, and (6R)-5,6,7,8-tetrahydro-L-biopterin are 12.7 microM, 14.4 microM, and 15.7 microM, respectively. These results suggest that mammalian GTP cyclohydrolase I may be regulated by its metabolic end products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
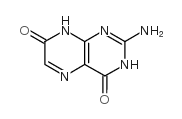 |
isoxanthopterin
CAS:529-69-1 |
C6H5N5O2 |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
Normalization of urinary pteridines by urine specific gravit...
2014-08-05 [Clin. Chim. Acta 435 , 42-7, (2014)] |
|
Direct radical scavenging activity of benzbromarone provides...
2015-01-01 [Biol. Pharm. Bull. 38(3) , 487-92, (2015)] |
|
Purification and partial characterization of xanthine oxidas...
1992-07-21 [Biochim. Biophys. Acta 1117(1) , 25-32, (1992)] |
|
Pterin detection using surface-enhanced Raman spectroscopy i...
2011-07-01 [J. Biomed. Opt. 16(7) , 077007, (2011)] |