Hydrogen abstraction ability of different aromatic nitroxides.
Elisabetta Damiani, Paola Astolfi, Massimo Benaglia, Angelo Alberti, Lucedio Greci
Index: Free Radic. Res. 38(1) , 67-72, (2004)
Full Text: HTML
Abstract
Indolinonic aromatic nitroxides have been shown to efficiently inhibit free radical mediated oxidation reactions in biological systems. Since all antioxidants also possess pro-oxidant activity, possibly through a hydrogen abstraction process from suitable substrates, the relative hydrogen abstraction abilities of these compounds were evaluated. Different hydrogen donors were reacted with an indolinic and two indolinonic nitroxides and the rates of hydrogen abstraction were determined using UV-Vis spectroscopy. From the data obtained, a structure-activity relationship was found. In addition, the hydrogen abstraction ability of these compounds was found to be much greater than that of the aliphatic nitroxide TEMPO, despite existing reports indicating that these two classes of compounds show similar antioxidant activities in biological systems.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
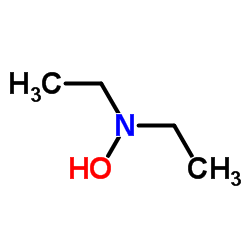 |
N,N-Diethylhydroxylamine
CAS:3710-84-7 |
C4H11NO |
|
The effect of diethylhydroxylamine on the incidence of tumor...
1984-01-01 [Drug Chem. Toxicol. 7(4) , 315-27, (1984)] |
|
N,N-diethylhydroxylamine: a new electron donor to photosyste...
1991-09-16 [Biochem. Biophys. Res. Commun. 179(2) , 1127-33, (1991)] |
|
A rare fatal case of silicone resin precursor (SH792) poison...
1991-03-01 [Am. J. Forensic Med. Pathol. 12(1) , 54-8, (1991)] |
|
Theoretical and spectroscopic study of the reaction of dieth...
2007-04-07 [Phys. Chem. Chem. Phys. 9(13) , 1629-34, (2007)] |
|
The photooxidation of diethylhydroxylamine by rose bengal in...
1993-07-01 [Photochem. Photobiol. 58(1) , 11-8, (1993)] |