Carbohydrate carbonyl mobility--the key process in the formation of alpha-dicarbonyl intermediates.
Oliver Reihl, Thorsten M Rothenbacher, Markus O Lederer, Wolfgang Schwack
Index: Carbohydr. Res. 339 , 1609-1618, (2004)
Full Text: HTML
Abstract
Covalently cross-linked proteins are among the major modifications caused by the advanced Maillard reaction. In the present study, the formation pathway of the dideoxyosone N6-(2,3-dihydroxy-5,6-dioxohexyl)-L-lysine is shown. To elucidate the formation of this glucose-derived dideoxyosone D-lactose (O-beta-D-galp-(1-->4)-D-glcp) and D-glucose-6-phosphate were incubated with lysine in the presence of the trapping reagent o-phenylenediamine (OPD). Synthesis and unequivocal structural characterization were reported for the quinoxalines of the dideoxyosones N6-(5,6-dihydroxy-2,3-dioxohexyl)-L-lysine and N6-(2,3-dihydroxy-4,5-dioxohexyl)-L-lysine, respectively. Additionally, dicarbonyl compounds derived from D-erythrose, D-glycero-D-mannoheptose, and D-gluco-L-talooctose were synthesized and structurally characterized.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
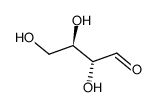 |
D-Erythrose
CAS:583-50-6 |
C4H8O4 |
|
Biosynthesis of l-Sorbose and l-Psicose Based on C-C Bond Fo...
2015-07-01 [Appl. Environ. Microbiol. 81 , 4284-94, (2015)] |
|
Identification of novel thermostable taurine-pyruvate transa...
2016-04-01 [Appl. Microbiol. Biotechnol. 100 , 3101-11, (2016)] |
|
Conformational study of the open-chain and furanose structur...
2012-09-01 [Carbohydr. Res. 358 , 96-105, (2012)] |
|
Computational study of mutarotation in erythrose and threose...
2011-12-27 [Carbohydr. Res. 346(18) , 2933-9, (2011)] |
|
Growth of organic microspherules in sugar-ammonia reactions.
2005-12-01 [Orig. Life Evol. Biosph. 35 , 523-536, (2005)] |