Metabolic response against sulfur-containing heterocyclic compounds by the lignin-degrading basidiomycete Coriolus versicolor.
H Ichinose, M Nakamizo, H Wariishi, H Tanaka
Index: Appl. Microbiol. Biotechnol. 58(4) , 517-26, (2002)
Full Text: HTML
Abstract
The fungal conversions of sulfur-containing heterocyclic compounds were investigated using the lignin-degrading basidiomycete Coriolus versicolor. The fungus metabolized a series of sulfur compounds--25 structurally related thiophene derivatives--via several different pathways. Under primary metabolic conditions, C. versicolor utilized thiophenes, such as 2-hydroxymethyl-, 2-formyl-, and 2-carboxyl-thiophenes, as a nutrient sulfur source for growth; thus, the fungus degraded these compounds more effectively in a non-sulfur-containing medium than in conventional medium. The product analysis revealed that several redox reactions, decarboxylation reactions, and C-S cleavage reactions were involved in the fungal conversion of non-aromatic thiophenes. On the other hand, benzothiophene (BT) and dibenzothiophene (DBT) skeletons were converted to water-soluble products. All the products and metabolic intermediates were more hydrophilic than the starting substrates. These metabolic actions seemed to be a chemical stress response against exogenously added xenobiotics. These metabolic reactions were optimized under ligninolytic conditions, also suggesting the occurrence of a fungal xenobiotic response. Furthermore, the fungus converted a series of BTs and DBTs via several different pathways, which seemed to be controlled by the chemical structure of the substrates. DBT, 4-methylDBT, 4, 6-dimethylDBT, 2-methylBT, and 7-methylBT were immediately oxidized to their S-oxides. BTs and DBTs with the hydroxymethyl substituent were converted to their xylosides without S-oxidation. Those with carboxyl and formyl substituents were reduced to form a hydroxymethyl group, then xylosidated. These observations strongly suggested the involvement of a fungal substrate-recognition and metabolic response mechanism in the metabolism of sulfur-containing heterocyclic compounds by C. versicolor.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
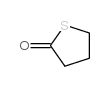 |
4-BUTYROTHIOLACTONE
CAS:1003-10-7 |
C4H6OS |
|
A Novel Synthesis of Poly(ester-alt-sulfide)s by the Ring-Op...
1998-07-01 [Macromolecules 31(15) , 4746-52, (1998)] |
|
Mechanism of hydrolysis and aminolysis of homocysteine thiol...
2006-05-15 [Chemistry 12(15) , 4144-52, (2006)] |
|
Structure-activity studies of fluoroalkyl-substituted gamma-...
1998-01-01 [Bioorg. Med. Chem. 6(1) , 43-55, (1998)] |
|
Alkyl-substituted gamma-butyrolactones act at a distinct sit...
1993-06-25 [Brain Res. 615(1) , 170-4, (1993)] |
|
Phytogrowth-inhibitory activities of sulfur-containing compo...
1993-08-01 [Biol. Pharm. Bull. 16(8) , 813-6, (1993)] |