A Novel Synthesis of Poly(ester-alt-sulfide)s by the Ring-Opening Alternating Copolymerization of Oxiranes with gamma-Thiobutyrolactone Using Quaternary Onium Salts or Crown Ether Complexes as Catalysts.
Nishikubo, Kameyama, Kawakami
Index: Macromolecules 31(15) , 4746-52, (1998)
Full Text: HTML
Abstract
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or in various organic solvents at 30-120 degreesC, in which quaternary onium salts having Cl- as a counteranion such as tetrabutylammonium chloride (TBAC) had higher activity than quaternary onium salts such as tetrabutylammonium bromide having Br- as a counteranion. It was also found that the alternate copolymer (polymer 1) of GPE with TBL was obtained selectively under different feed ratios of GPE and TBL, although ring-opening homopolymerizations of GPE and TBL did not proceed. Copolymerizations of various oxiranes such as butyl glycidyl ether, styrene oxide, and 1,2-hexene oxide with TBL catalyzed by TBAC also proceeded, and the corresponding poly(ester-alt-sulfide)s (polymers 2, 3, and 4) were obtained under the same conditions as for the synthesis of polymer 1.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
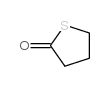 |
4-BUTYROTHIOLACTONE
CAS:1003-10-7 |
C4H6OS |
|
Metabolic response against sulfur-containing heterocyclic co...
2002-03-01 [Appl. Microbiol. Biotechnol. 58(4) , 517-26, (2002)] |
|
Mechanism of hydrolysis and aminolysis of homocysteine thiol...
2006-05-15 [Chemistry 12(15) , 4144-52, (2006)] |
|
Structure-activity studies of fluoroalkyl-substituted gamma-...
1998-01-01 [Bioorg. Med. Chem. 6(1) , 43-55, (1998)] |
|
Alkyl-substituted gamma-butyrolactones act at a distinct sit...
1993-06-25 [Brain Res. 615(1) , 170-4, (1993)] |
|
Phytogrowth-inhibitory activities of sulfur-containing compo...
1993-08-01 [Biol. Pharm. Bull. 16(8) , 813-6, (1993)] |