Introduction of alpha-hydroxymethyamino acid residues in substrate specificity P1 position of trypsin inhibitor SFTI-1 from sunflower seeds retains its activity.
Ewa Zabłotna, Agnieszka Kret, Anna Jaśkiewicz, Aleksandra Olma, Mirosław T Leplawy, Krzysztof Rolka
Index: Biochem. Biophys. Res. Commun. 340(3) , 823-8, (2006)
Full Text: HTML
Abstract
In many complexes formed by serine proteinases and their inhibitors, the hydroxyl group provided by water molecule or by the inhibitor Ser residue is located close to the inhibitor P1-P1' reactive site. In order to investigate the role of this group, we synthesized analogues of trypsin inhibitor SFTI-1 isolated from the seeds of sunflower modified in P1 by alpha-hydroxymethylserine (HmSer) and both enantiomers of alpha-hydroxymethylvaline (HmVal). All the synthesized analogues inhibited bovine beta-trypsin and human leukocyte elastase. SFTI-1 analogues with HmVal and HmSer appear to be potent inhibitors of bovine beta-trypsin, whereas [Val5]SFTI-1 is practically inactive. Also trypsin inhibitory activity of [Ser5]SFTI-1 is significantly lower. Since the electrostatic interaction between protonated epsilon-NH2 group of the inhibitor P1 position and beta-carboxylate of trypsin Asp189 is the main driving force for interaction of both molecules, the results obtained are very interesting. We believe that these SFTI-1 analogues belong to a novel class of serine proteinase inhibitors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
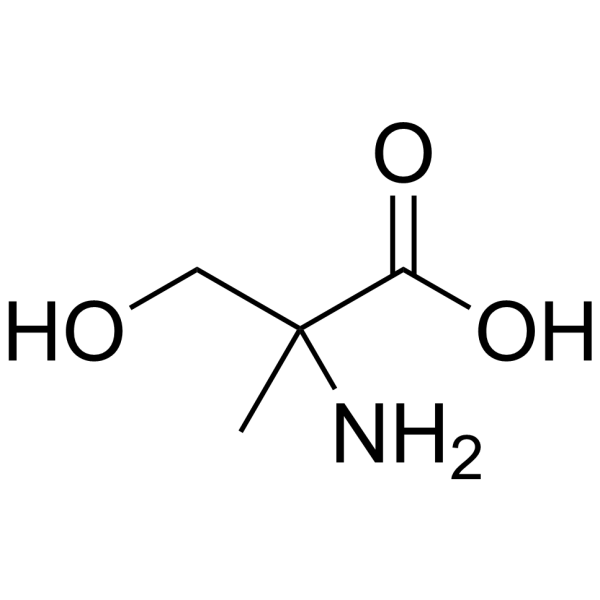 |
2-Methyl-L-serine
CAS:5424-29-3 |
C4H9NO3 |
|
Drug design, in vitro pharmacology, and structure-activity r...
2009-08-27 [J. Med. Chem. 52 , 5093-107, (2009)] |
|
The requirement of phosphorylation on a threonine residue in...
1993-09-01 [J. Steroid Biochem. Mol. Biol. 46(3) , 337-47, (1993)] |
|
Control of chemoselectivity in Dieckmann ring closures leadi...
2011-10-07 [Org. Biomol. Chem. 9 , 6663, (2011)] |
|
Synthesis, NMR spectra and function of peptides with alpha-m...
1992-10-01 [Acta Chem. Scand. 46(10) , 989-93, (1992)] |
|
Conformational studies on host-guest peptides containing chi...
1988-11-01 [Int. J. Pept. Protein Res. 32(5) , 344-51, (1988)] |