Synthetic transformation of ptychantin into forskolin and 1,9-dideoxyforskolin.
Hisahiro Hagiwara, Fumihide Takeuchi, Masaru Kudou, Takashi Hoshi, Toshio Suzuki, Toshihiro Hashimoto, Yoshinori Asakawa
Index: J. Org. Chem. 71(12) , 4619-24, (2006)
Full Text: HTML
Abstract
Forskolin (1), a highly oxygenated labdane diterpenoid and an activator of adenylate cyclase, has been synthesized in 12 steps and 12% overall yield from ptychantin A (4), which has been isolated from liverwort Ptychanthus striatus in good yield. The 1alpha-hydroxy group was furnished by stereoselective reduction of the corresponding carbonyl group by sodium in t-BuOH. The 9alpha-hydroxy group was introduced stereoselectively by epoxidation of delta(9.11)-enolether. 1,9-Dideoxyforskolin (2), an inhibitor of glucose transporter, has been synthesized in 8 steps and 37% overall yield. The hydroxy group at C-1 was removed by solid-state thicarbonylimidazolation and subsequent radical cleavage.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
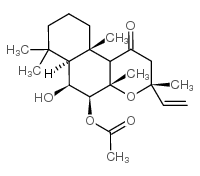 |
1,9-Dideoxyforskolin
CAS:64657-18-7 |
C22H34O5 |
|
Roles of calcium/calmodulin-dependent kinase II in long-term...
2014-01-01 [PLoS ONE 9(9) , e107442, (2014)] |
|
Inhibition of Akt reverses the acquired resistance to sorafe...
2014-06-01 [Mol. Cancer Ther. 13(6) , 1589-98, (2014)] |
|
The expression and regulation of depolarization-activated K+...
2001-04-01 [Pflugers Arch. 442(1) , 49-56, (2001)] |
|
Effect of protein kinase A-induced phosphorylation on the ga...
1999-07-01 [Biophys. J. 77(1) , 204-16, (1999)] |
|
Volume sensitive efflux of taurine in HEK293 cells overexpre...
2000-04-17 [Biochim. Biophys. Acta 1496(2-3) , 252-60, (2000)] |