| Structure | Name/CAS No. | Articles |
|---|---|---|
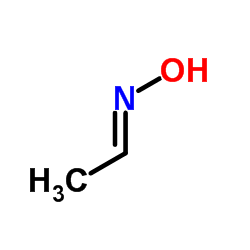 |
Acetaldoxime
CAS:107-29-9 |
|
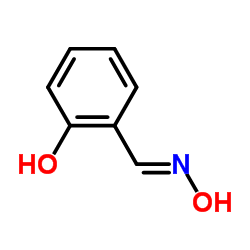 |
salicylaldehyde, oxime
CAS:94-67-7 |