Chemoenzymatic synthesis and chemical recycling of poly(ester-urethane)s.
Hiroto Hayashi, Yoshio Yanagishita, Shuichi Matsumura
Index: Int. J. Mol. Sci. 12 , 5490-507, (2011)
Full Text: HTML
Abstract
Novel poly(ester-urethane)s were prepared by a synthetic route using a lipase that avoids the use of hazardous diisocyanate. The urethane linkage was formed by the reaction of phenyl carbonate with amino acids and amino alcohols that produced urethane-containing diacids and hydroxy acids, respectively. The urethane diacid underwent polymerization with polyethylene glycol and α,ω-alkanediols and also the urethane-containing hydroxy acid monomer was polymerized by the lipase to produce high-molecular-weight poly(ester-urethane)s. The periodic introduction of ester linkages into the polyurethane chain by the lipase-catalyzed polymerization afforded chemically recyclable points. They were readily depolymerized in the presence of lipase into cyclic oligomers, which were readily repolymerized in the presence of the same enzyme. Due to the symmetrical structure of the polymers, poly(ester-urethane)s synthesized in this study showed higher T(m), Young's modulus and tensile strength values.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
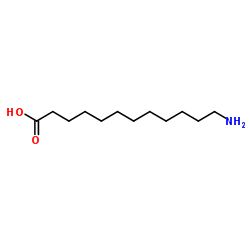 |
12-Aminododecanoic acid
CAS:693-57-2 |
C12H25NO2 |
|
Structure-activity relationship study on a simple cationic p...
2005-10-20 [J. Med. Chem. 48 , 6741-9, (2005)] |
|
Development of 68Ga-labeled fatty acids for their potential ...
2014-06-15 [J. Labelled Comp. Radiopharm. 57(7) , 463-9, (2014)] |
|
Multi-dye theranostic nanoparticle platform for bioimaging a...
2012-01-01 [Int. J. Nanomedicine 7 , 2739-50, (2012)] |
|
The influence of montmorillonite clay reinforcement on the p...
2006-11-01 [J. Dent. 34(10) , 802-10, (2006)] |
|
The impact of montmorillonite clay addition on the in vitro ...
2007-04-01 [J. Dent. 35(4) , 309-17, (2007)] |