RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition.
Simone Lipinski, Nils Grabe, Gunnar Jacobs, Susanne Billmann-Born, Andreas Till, Robert Häsler, Konrad Aden, Maren Paulsen, Alexander Arlt, Lars Kraemer, Nina Hagemann, Kai Sven Erdmann, Stefan Schreiber, Philip Rosenstiel
Index: Proc. Natl. Acad. Sci. U. S. A. 109(52) , 21426-31, (2012)
Full Text: HTML
Abstract
The intracellular nucleotide-binding oligomerization domain-2 (NOD2) receptor detects bacteria-derived muramyl dipeptide (MDP) and activates the transcription factor NF-κB. Here we describe the regulatome of NOD2 signaling using a systematic RNAi screen. Using three consecutive screens, we identified a set of 20 positive NF-κB regulators including the known pathway members RIPK2, RELA, and BIRC4 (XIAP) as well as FRMPD2 (FERM and PDZ domain-containing 2). FRMPD2 interacts with NOD2 via leucine-rich repeats and forms a complex with the membrane-associated protein ERBB2IP. We demonstrate that FRMPD2 spatially assembles the NOD2-signaling complex, hereby restricting NOD2-mediated immune responses to the basolateral compartment of polarized intestinal epithelial cells. We show that genetic truncation of the NOD2 leucine-rich repeat domain, which is associated with Crohn disease, impairs the interaction with FRMPD2, and that intestinal inflammation leads to down-regulation of FRMPD2. These results suggest a structural mechanism for how polarity of epithelial cells acts on intestinal NOD-like receptor signaling to mediate spatial specificity of bacterial recognition and control of immune responses.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
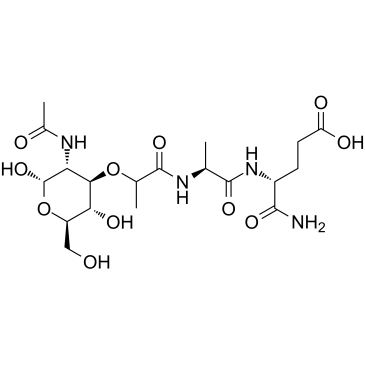 |
Ac-muramyl-Ala-D-Glu-NH2
CAS:53678-77-6 |
C19H32N4O11 |
|
Enhanced anti-tumor immune responses and delay of tumor deve...
2015-01-01 [Breast Cancer Res. 17 , 48, (2015)] |
|
The crosstalk between TLR2 and NOD2 in Aspergillus fumigatus...
2015-04-01 [Mol. Immunol. 64(2) , 235-43, (2015)] |
|
Sensing of commensal organisms by the intracellular sensor N...
2012-08-24 [Immunity 37(2) , 326-38, (2012)] |
|
Mifamurtide in metastatic and recurrent osteosarcoma: a pati...
2014-02-01 [Pediatr. Blood Cancer 61(2) , 238-44, (2014)] |
|
Mifamurtide for high-grade, resectable, nonmetastatic osteos...
2013-12-01 [Value Health 16(8) , 1123-32, (2013)] |