Recognition of Streptococcus pneumoniae and muramyl dipeptide by NOD2 results in potent induction of MMP-9, which can be controlled by lipopolysaccharide stimulation.
Marloes Vissers, Yvonne Hartman, Laszlo Groh, Dirk J de Jong, Marien I de Jonge, Gerben Ferwerda
Index: Infect. Immun. 82(12) , 4952-8, (2014)
Full Text: HTML
Abstract
Matrix metallopeptidase 9 (MMP-9) is a protease involved in the degradation of extracellular matrix collagen. Evidence suggests that MMP-9 is involved in pathogenesis during Streptococcus pneumoniae infection. However, not much is known about the induction of MMP-9 and the regulatory processes involved. We show here that the Gram-positive bacteria used in this study induced large amounts of MMP-9, in contrast to the Gram-negative bacteria that were used. An important pathogen-associated molecular pattern (PAMP) for Gram-positive bacteria is muramyl dipeptide (MDP). MDP is a very potent inducer of MMP-9 and showed a dose-dependent MMP-9 induction. Experiments using peripheral blood mononuclear cells (PBMCs) from Crohn's disease patients with nonfunctional NOD2 showed that MMP-9 induction by Streptococcus pneumoniae and MDP is NOD2 dependent. Increasing amounts of lipopolysaccharide (LPS), an important PAMP for Gram-negative bacteria, resulted in decreasing amounts of MMP-9. Moreover, the induction of MMP-9 by MDP could be counteracted by simultaneously adding LPS. The inhibition of MMP-9 expression by LPS was found to be regulated posttranscriptionally, independently of tissue inhibitor of metalloproteinase 1 (TIMP-1), an endogenous inhibitor of MMP-9. Collectively, these data show that Streptococcus pneumoniae is able to induce large amounts of MMP-9. These high MMP-9 levels are potentially involved in Streptococcus pneumoniae pathogenesis. Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
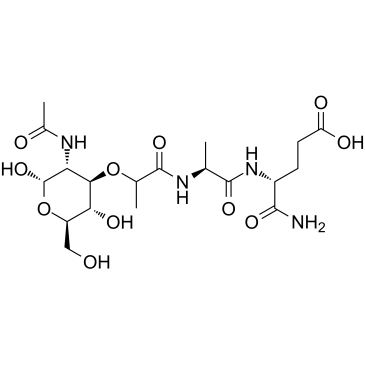 |
Ac-muramyl-Ala-D-Glu-NH2
CAS:53678-77-6 |
C19H32N4O11 |
|
Enhanced anti-tumor immune responses and delay of tumor deve...
2015-01-01 [Breast Cancer Res. 17 , 48, (2015)] |
|
The crosstalk between TLR2 and NOD2 in Aspergillus fumigatus...
2015-04-01 [Mol. Immunol. 64(2) , 235-43, (2015)] |
|
Sensing of commensal organisms by the intracellular sensor N...
2012-08-24 [Immunity 37(2) , 326-38, (2012)] |
|
Mifamurtide in metastatic and recurrent osteosarcoma: a pati...
2014-02-01 [Pediatr. Blood Cancer 61(2) , 238-44, (2014)] |
|
Mifamurtide for high-grade, resectable, nonmetastatic osteos...
2013-12-01 [Value Health 16(8) , 1123-32, (2013)] |