D Ross, P B Farmer, A Gescher, J A Hickman, M D Threadgill
Index: Biochem. Pharmacol. 32(11) , 1773-81, (1983)
Full Text: HTML
The stability of metabolically-generated N-(hydroxymethyl) compounds was investigated using a series of N-methylbenzamides as model substrates. N-(Hydroxymethyl)-benzamide was characterized as a major metabolite of N-methylbenzamide in vitro, and was also identified as a urinary metabolite of N-methylbenzamide. N-(Hydroxymethyl) compounds were also found as metabolites of 4-chloro-N-methylbenzamide and 4-t-butyl-N-methylbenzamide in vitro. Thus substitution in the 4-position of the phenyl ring of derivatives of N-(hydroxymethyl)-benzamide did not affect their stability sufficiently to cause degradation to formaldehyde under the conditions used. N-(Hydroxymethyl)-N-methylbenzamide was identified as a metabolite of N,N-dimethylbenzamide in vitro. However, N-(hydroxymethyl)-N-methylbenzamide was less stable than N-(hydroxymethyl)-benzamide under alkaline conditions. Furthermore, N-(hydroxymethyl)-N-methylbenzamide, unlike N-(hydroxymethyl)-benzamide and its 4-substituted derivatives, was positive in the colorimetric assay for formaldehyde, presumably because of its degradation to produce formaldehyde. Thus substitution on the nitrogen atom which bears the methyl group in N-methylbenzamide markedly affected the stability of the N-methylol produced during oxidative metabolism. N-Formylbenzamide was identified as a metabolite of N-methylbenzamide in suspensions of mouse hepatocytes and also in vivo. The mechanism for its production probably involves the generation of N-(hydroxymethyl)-benzamide.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
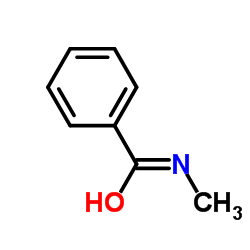 |
1MVR
CAS:613-93-4 |
C8H9NO | |
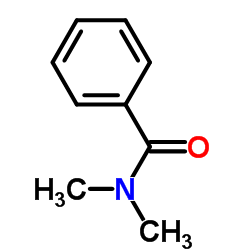 |
1N1&VR
CAS:611-74-5 |
C9H11NO |
|
Thermodynamic analysis of the binding of aromatic hydroxamic...
2001-11-20 [Biochemistry 40(46) , 13980-9, (2001)] |
|
N-Methylanilide and N-methylbenzamide derivatives as phospho...
2013-10-01 [Bioorg. Med. Chem. 21(19) , 6053-62, (2013)] |
|
Molecular electrostatic potential of orthopramides: implicat...
1986-05-01 [J. Med. Chem. 29(5) , 600-6, (1986)] |
|
The microsomal demethylation of N,N-dimethylbenzamides. Subs...
1992-08-18 [Biochem. Pharmacol. 44(4) , 651-8, (1992)] |
|
Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4...
2009-09-15 [Bioorg. Med. Chem. Lett. 19(18) , 5464-8, (2009)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved