Biosynthesis of calystegines: 15N NMR and kinetics of formation in root cultures of Calystegia sepium.
Yvonne Scholl, Bernd Schneider, Birgit Dräger
Index: Phytochemistry 62(3) , 325-32, (2003)
Full Text: HTML
Abstract
Calystegines are nortropane alkaloids bearing between three and five hydroxyl groups in various positions. [15N]Tropinone was administered to root cultures of Calystegia sepium and the incorporation into calystegines was followed. Increase of label in calystegines was measured by one-dimensional 15N NMR and inverse-detected 2D NMR techniques. The results show that tropinone and pseudotropine are metabolites in the biosynthetic pathway of calystegines. The velocity of calystegine accumulation was followed kinetically by transfer of root cultures from 15N-enriched medium to 14N-medium and analysis by GC-MS. A constant calystegine formation with no interference by excretion or degradation was observed. A biosynthetic rate for individual calystegines at each time point was calculated, the maximum was 0.4 mg/day/g of biomass. This allowed the velocity of individual biosynthetic steps to be estimated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
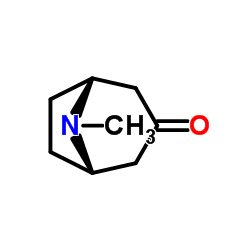 |
Tropinone
CAS:532-24-1 |
C8H13NO |
|
Toxicity of field bindweed (Convolvulus arvensis) to mice.
1995-10-01 [Vet. Hum. Toxicol. 37(5) , 452-4, (1995)] |
|
Synthesis and applications of masked oxo-sulfinamides in asy...
2012-07-14 [Org. Biomol. Chem. 10(26) , 5021-31, (2012)] |
|
Molecular dissection of tropisetron, an alpha7 nicotinic ace...
2005-04-22 [Neurosci. Lett. 378(3) , 140-4, (2005)] |
|
Total synthesis of (±)-parvineostemonine.
2012-10-01 [Chem. Asian J. 7(10) , 2199-202, (2012)] |
|
An approach for synthesis of tropinone analogue N-substitute...
2008-01-01 [Acta Pol. Pharm. 65(6) , 749-51, (2008)] |