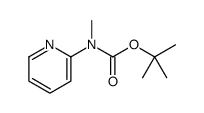
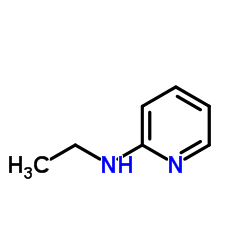
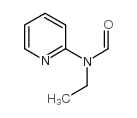
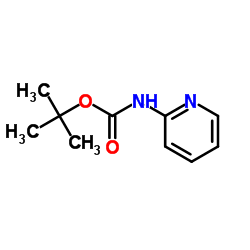
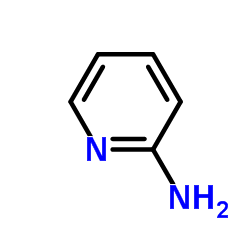
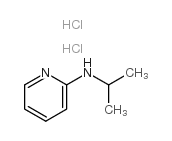
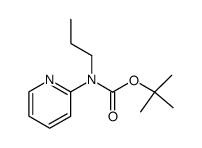
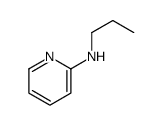
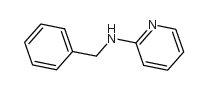
|
~96% |
|
~94% |
|
~88% |
|
~% |
|
~85% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~97% |