[Organ distribution of ruthenocenyl biogenic amine derivatives--radiodiagnostic agents for the adrenals and ovaries?].
M Wenzel, P Asindraza
Index: Strahlenther. Onkol. 162(1) , 51-6, (1986)
Full Text: HTML
Abstract
The organ distribution of 103Ru labelled ruthenocenyl derivatives of tyramine, histamine, benzylamine, phenylethylamine and homoveratrylamine were measured in rats. The derivatives of tyramine, histamine and benzylamine showed a high affinity for the adrenal and ovar. Adrenal/muscle ratios up to 2000:1 were gained but only if the dose was administered i.v. and was below 0.1 mumol/kg. The ruthenocenyl derivatives of tyramine labelled with 103Ru in the ruthenocene moiety or with 14C in the tyramine moiety showed a parallel distribution pattern but completely different from the distribution of 103RuCl3. This indicates that the tyramine derivatives are not destroyed in the body yielding Ru-ions. The advantages of the ruthenocenyl derivatives in comparison with the known amphetamine derivatives labelled with radioactive iodine are discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
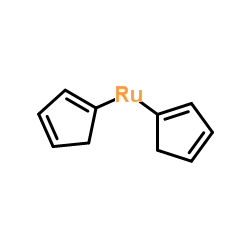 |
Di-1,3-cyclopentadien-1-ylruthenium
CAS:1287-13-4 |
C10H10Ru |
|
Selective estrogen receptor modulators in the ruthenocene se...
2005-04-21 [J. Med. Chem. 48 , 2814-21, (2005)] |
|
Group 8 metallocenes as bulky functional groups in glucopyra...
2013-01-10 [Carbohydr. Res. 365 , 26-31, (2013)] |
|
Structural investigation of the interactions of biotinylruth...
2013-06-25 [Chem. Biol. Interact. 204(1) , 6-12, (2013)] |
|
Adrenal-affinity of ruthenocenyl-derivatives of biogenic ami...
1985-01-01 [Int. J. Nucl. Med. Biol. 12(1) , 1-2, (1985)] |
|
[Enzymatic hydroxylation of ruthenocene and osmocene by live...
1982-01-01 [Z. Naturforsch., C, J. Biosci. 37(1-2) , 136-8, (1982)] |