| Structure | Name/CAS No. | Articles |
|---|---|---|
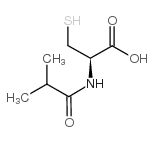 |
i-but-cys-oh
CAS:124529-02-8 |
|
 |
n-isobutyryl-d-cysteine
CAS:124529-07-3 |