| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
tricosanoic acid tryptamide
CAS:152766-93-3 |
|
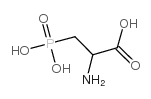 |
DL-AP3
CAS:20263-06-3 |