Use of achiral/chiral SFC/MS for the profiling of isomeric cinnamonitrile/hydrocinnamonitrile products in chiral drug synthesis.
A J Alexander, A Staab
Index: Anal. Chem. 78(11) , 3835-8, (2006)
Full Text: HTML
Abstract
A directly coupled achiral/chiral SFC/MS method has been developed for the profiling of a three-step stereoselective synthesis of cinnamonitrile and hydrocinnamonitrile intermediates. Semipurified reaction mixtures were screened in one step to determine the diastereomeric/enantiomeric composition of the final product as well as to identify any remaining E/Z isomers present from the starting material. The coupled achiral/chiral column combination was found to significantly enhance the separation of both enantiomers and diastereomers, without adding significantly to the overall analysis time. This analytical technique should prove to be generally useful for the profiling of isomeric reaction products in chiral drug synthesis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
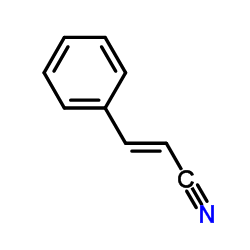 |
trans-Cinnamonitrile
CAS:1885-38-7 |
C9H7N |
|
The spectroscopy and photochemistry of quinioline structural...
2015-08-21 [J. Chem. Phys. 143 , 074304, (2015)] |
|
Reversible targeting of noncatalytic cysteines with chemical...
2012-05-01 [Nat. Chem. Biol. 8 , 471-6, (2012)] |
|
Formation of mercapturic acids from acrylonitrile, crotononi...
1981-01-01 [Drug Metab. Dispos. 9(3) , 246-9, (1981)] |
|
Synthesis and use of an in-solution ratiometric fluorescent ...
2007-01-01 [Nat. Protoc. 2(1) , 227-36, (2007)] |
|
Direct oxidative coupling of benzenes with acrylonitriles to...
2010-09-21 [Org. Biomol. Chem. 8(18) , 4071-3, (2010)] |