| Structure | Name/CAS No. | Articles |
|---|---|---|
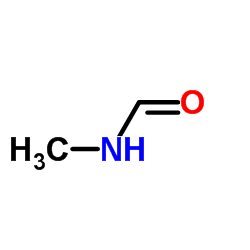 |
N-methylformamide
CAS:123-39-7 |
|
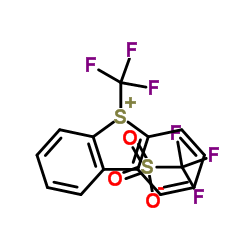 |
S-(Trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate
CAS:129946-88-9 |
|
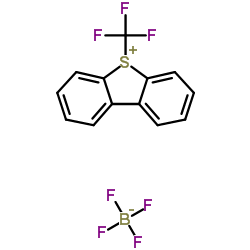 |
S-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate
CAS:131880-16-5 |