The effect of perchlorate ions on a pyridine-based microgel
Joseph P. Cook, D. Jason Riley, Joseph P. Cook, D. Jason Riley
Index: Adv. Colloid Interface Sci. 147-148 , 67-73, (2009)
Full Text: HTML
Abstract
The effect of perchlorate ions as counterions in the acid-induced swelling of poly(2-vinylpyridine) microgel particles was investigated. The p H was modified with perchloric, hydrochloric and hydrobromic acids and the particle diameter measured using dynamic light scattering and the viscosity monitored using a capillary viscometer. The microgel particles were found to have a lower apparent p K a with perchloric acid, 4.10 compared to 4.70 with hydrochloric and hydrobromic acids. As a result the particles swell at a lower p H and they also swell to a lower maximum diameter with perchloric acid, 681 ± 8 nm compared to 751 ± 15 nm for hydrochloric and hydrobromic acids. The swelling transition is also continuous with perchloric acid, whereas it has been identified as first order with hydrochloric acid. Similar variations were found in the viscosity using the different acids. The differences can be accounted for by the dehydrating and cross-linking nature of the perchlorate ion.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
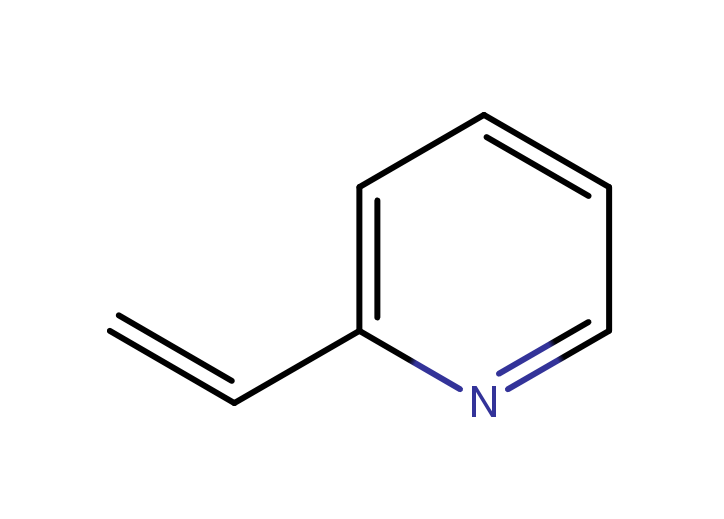 |
poly(2-vinylpyridine)
CAS:25014-15-7 |
C7H7N |
|
Core-crosslinked compartmentalized cylinders.
2011-01-01 [Nanoscale 3(1) , 288-97, (2011)] |
|
Fluorescent nanoparticles stabilized by poly(ethylene glycol...
2010-07-06 [Langmuir 26(13) , 10684-92, (2010)] |
|
Tuning the adhesion of silica microparticles to a poly(2-vin...
2012-11-06 [Langmuir 28(44) , 15555-65, (2012)] |
|
Adsorption of 5-sodiosulfoisophthalic acids from aqueous sol...
2010-03-15 [J. Hazard. Mater. 175(1-3) , 111-6, (2010)] |
|
In situ metallization of patterned polymer brushes created b...
2013-04-19 [Nanotechnology 24(15) , 155602, (2013)] |