Compactness of the molten globule in comparison to unfolded states as observed by size-exclusion chromatography.
E Zerovnik, R Jerala, N Poklar, L Kroon-Zitko, V Turk
Index: Biochim. Biophys. Acta 1209(1) , 140-3, (1994)
Full Text: HTML
Abstract
The volumes of elution of denatured states of four proteins at high urea (8 M) and ethylurea (6 M) concentration were determined. They were found equally unfolded in both solvents. The volumes of elution of the unfolded states were compared to those of the native states and of some molten globule intermediates. It has been shown that the protein proteinase inhibitor stefin B, exhibits 'molten globule'-like properties on acid denaturation. The high salt acidic intermediate (a molten globule) as well as the native state of stefin B eluted as dimers, at 18 degrees C. On thermal denaturation above 42 degrees C, the intermediate dissociated into compact monomers. The more stable stefin A, which is monomeric and does not transform into molten globule intermediates under similar perturbing conditions, was always used for comparison. The states of both, stefin A and B in 50% methanol were found to be monomeric and of native-like compactness.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
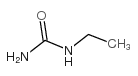 |
Ethylurea
CAS:625-52-5 |
C3H8N2O |
|
Solvation of human serum albumin in aqueous alkylurea soluti...
1993-10-01 [Int. J. Pept. Protein Res. 42(4) , 320-5, (1993)] |
|
Inhibition of HIV-1 infection by alkylureas.
1991-12-01 [AIDS 5(12) , 1447-52, (1991)] |
|
Neoplasia induced in male rats fed lead acetate, ethyl urea,...
1985-01-01 [Toxicol. Pathol. 13(1) , 50-7, (1985)] |
|
Mutagenic effects of nitrogen dioxide combined with methylur...
1981-03-01 [Mutat. Res. 88(3) , 281-90, (1981)] |
|
Comparison of nicotinamide, ethylurea and polyethylene glyco...
1997-10-01 [Chem. Pharm. Bull. 45(10) , 1688-93, (1997)] |