Quinone oxidoreductase-2-mediated prodrug cancer therapy.
Mark R Middleton, Richard Knox, Emma Cattell, Udo Oppermann, Rachel Midgley, Raghib Ali, Tim Auton, Roshan Agarwal, David Anderson, Debashis Sarker, Ian Judson, Tsuyoshi Osawa, Victoria J Spanswick, Scot Davies, John A Hartley, David J Kerr
Index: Sci. Transl. Med. 2(40) , 40ra50, (2010)
Full Text: HTML
Abstract
DNA-damaging agents are widely used in cancer treatment despite their lack of tumor specificity. Human NQO2 (quinone oxidoreductase-2) is an atypical oxidoreductase because no endogenous electron donor has been identified to date. The enzyme converts CB1954 [5-(aziridin-1-yl)-2,4-dinitrobenzamide], in the presence of the synthetic nicotinamide cofactor analog EP0152R, to a cytotoxic bifunctional alkylating agent. NQO2 activity in hepatocellular tumor tissue is higher than that in other cancer types by a factor of 6 and higher than that in bone marrow by a factor of 20. Structural data from x-ray crystallography and nuclear magnetic resonance spectroscopy allowed us to construct a model of CB1954 and EP0152R binding to NQO2, which suggested an optimal infusion schedule for a phase I trial combining the two agents. Thirty-two patients were treated, and diarrhea and serum transaminase concentrations defined a maximum tolerated dose for the drug combination. There was a clear pharmacokinetic interaction, with EP0152R inducing a marked increase in clearance of CB1954, in keeping with model predictions. We detected DNA interstrand cross-links caused by nitroreduced CB1954 in tumor biopsies from treated patients, demonstrating that the activated prodrug exerts its cytotoxic properties through DNA base alkylation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
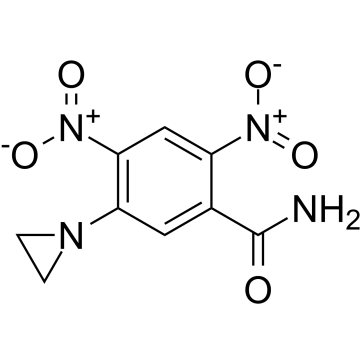 |
CB 1954
CAS:21919-05-1 |
C9H8N4O5 |
|
An unusually cold active nitroreductase for prodrug activati...
2012-06-01 [Bioorg. Med. Chem. 20(11) , 3540-50, (2012)] |
|
uvrB gene deletion enhances SOS chromotest sensitivity for n...
2010-10-01 [J. Biotechnol. 150(1) , 190-4, (2010)] |
|
CB 1954: from the Walker tumor to NQO2 and VDEPT.
2003-01-01 [Curr. Pharm. Des. 9(26) , 2091-104, (2003)] |
|
Colloidal gold modified with a genetically engineered nitror...
2011-12-06 [Langmuir 27(23) , 14300-7, (2011)] |
|
Antivector and tumor immune responses following adenovirus-d...
2009-11-01 [Hum. Gene Ther. 20(11) , 1249-58, (2009)] |