Test therapy in the treatment of generalized anxiety disorders with low dose fluspirilene.
C Wurthmann, E Klieser, E Lehmann, U Pester
Index: Prog. Neuropsychopharmacol. Biol. Psychiatry 19(6) , 1049-60, (1995)
Full Text: HTML
Abstract
1. The present double-blind study was designed to determine under three different conditions (0.5 mg, 1.0 mg, 1.5 mg per week) whether response or non-response within a two-week test-therapy predicts clinical outcome after 6 weeks of fluspirilene treatment in generalized anxiety disorders. 2. 106 outpatients entered the study. The period of observation was 6 weeks. 3. Confirming previous reports of their study group the authors found a significant reduction of anxiety in all treatment groups. However, this effect was mainly observed with the highest dose administered. The main finding of the study is that there is a significant correlation between initial response after 2 weeks of test therapy and therapeutic success after 6 weeks in fluspirilene treatment of generalized anxiety disorders. 4. Decreases in somatic anxiety, psychic anxiety and Hamilton-total-score within the first 2 weeks correlate with the baseline-to-week 6 decreases of the corresponding item and with the global clinical assessment of efficacy after 6 weeks. 5. By means of test therapy patients with an unfavourable outcome are identified and, if medication is discontinued, are prevented from an ineffective longterm treatment.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
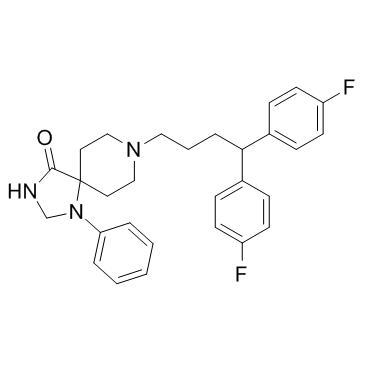 |
fluspirilene
CAS:1841-19-6 |
C29H31F2N3O |
|
Small molecule regulators of autophagy identified by an imag...
2007-11-27 [Proc. Natl. Acad. Sci. U. S. A. 104 , 19023-8, (2007)] |
|
Determination of fluspirilene in human plasma by liquid chro...
1998-12-18 [J. Chromatogr. A. 828(1-2) , 219-27, (1998)] |
|
A crystallographic and molecular modeling study of butyrophe...
1998-12-01 [J. Pharm. Sci. 87(12) , 1496-501, (1998)] |
|
Grief hallucinations: true or pseudo? Serious or not? An inq...
2002-01-01 [Psychopathology 35(5) , 296-302, (2002)] |
|
Stable expression and characterization of the human brain po...
1997-06-27 [Brain Res. 761(1) , 42-50, (1997)] |